Schaeffer Center White Paper Series | DOI: 10.25549/4rf9-kh77
Policy Context
Obesity affects almost half of U.S. adults and is associated with increased disability, disease and pain that reduces quality of life and increases future healthcare spending.
Yet issues with the safety and effectiveness of earlier treatments in the 1990s led Congress to specifically prohibit Medicare from covering obesity treatments in 2006 as part of the Medicare Prescription Drug, Improvement, and Modernization Act (MMA). Most private insurers also do not cover obesity treatments, despite the fact that new treatments are proving to be highly safe and effective. Bills designed to overturn Medicare’s coverage moratorium have been introduced in each Congress for the last 10 years. However, there is increasing concern about the impact that broad Medicare coverage would have on Part D spending and overall Medicare solvency.
Our analysis shows that reducing obesity rates would also decrease the incidence of many related conditions, such as heart disease and diabetes, that each independently raise medical spending and reduce quality of life. All told, in the first 10 years alone, Medicare coverage of weight-loss therapies would save the program $175 billion to $245 billion, depending on whether private insurance also covers the treatments. Over 60% of these savings would accrue to Medicare Part A by reducing hospital inpatient care demands and the demand for skilled nursing care. Given these findings, policymakers should consider the societal benefits of lifting the moratorium on Medicare coverage for weight-loss drugs and enable Medicare to work with manufacturers to create reimbursement solutions that provide broad access to new treatments.
Key Takeaways
- New, highly-effective treatments for obesity are available, yet federal law constrains access to only 1% of Americans eligible for treatment.
- The cumulative social benefits from Medicare coverage for new obesity treatments over the next 10 years would reach almost $1 trillion, or roughly $100 billion per year.
- Medicare coverage of weight-loss therapies would save federal taxpayers as much as $245 billion in the first 10 years of coverage alone, if private insurers were to follow Medicare’s lead.
A press release covering this white paper’s findings is available here.
Abstract
Obesity is among the most pressing public health concerns in the United States. Government agencies and medical associations have raised concerns about obesity trends, but they show no sign of abating. Although effective obesity treatments are available, access remains a challenge—Medicare and most private insurance plans do not cover them. Bills designed to overturn Medicare’s coverage moratorium have been introduced in each Congress over the last 10 years. However, some policymakers are concerned about the impact that broad Medicare coverage would have on Part D spending and overall Medicare solvency.
In this paper, we model the potential social benefits—and medical cost offsets—from passing the Treat and Reduce Obesity Act (TROA) to ensure universal Medicare access to the newer class of weight-loss drugs. We use the Future Adult Model, a well-established microsimulation model, to estimate potential social benefits—and medical cost offsets—of universal Medicare access to weight-loss medications.
Our analysis demonstrates that treating obesity at current efficacy rates would generate substantial benefits to society. The cumulative social benefits generated from Medicare coverage for new obesity treatments over the next 10 years would reach almost $1 trillion, or roughly $100 billion per year. If private insurance were also to cover weight-loss drugs, the benefits would rise further. We also show that passing TROA would generate significant cost offsets for Medicare. In the first 10 years alone, covering weight-loss therapies would save Medicare $175 billion to $245 billion, depending on whether private insurance joins Medicare in providing coverage for younger populations.
Introduction
Obesity is one of the most pressing public health issues in America. Even before the onset of COVID, researchers highlighted the “striking” association between obesity and mortality—a finding that persists even among those with access to insurance and quality care.1,2 Beyond its mortality effects, excess weight is associated with disability, disease and pain that reduce quality of life and increase future healthcare spending.3 Indeed, obesity poses a greater challenge to fiscal solvency than smoking.4
Government agencies and medical associations have been raising these concerns for over two decades, but with little impact on the overall trend.5 In a controversial 2013 vote, the American Medical Association (AMA) recognized obesity as a disease, and sought to raise awareness about the link between obesity and its sequelae—including diabetes, heart disease, hypertension, stroke and cancer. The AMA’s hope was that defining obesity as a disease might change the public perception that obesity is a lifestyle choice and medical treatments are unnecessary.6 However, a decade later, obesity prevalence continues to increase at alarming rates, and most Americans still lack access to effective treatments.
The issue is not the absence of weight-management treatment options. Five drugs approved by the Food and Drug Administration (FDA) are currently on the market and can help reduce body weight by 6% to 16%; a sixth drug is expected later this year that, trial data suggest, leads to an average of 20% weight loss. These are the most effective pharmaceutical treatments to date,7 yet only 1% of Americans eligible for treatment have access to them.8,9 Bariatric surgery is another treatment option available for patients with BMI > 40 that can reduce BMI by 26%, but only about 1% of eligible patients receive the surgery due to the potential risks, out-of-pocket costs and potential for long-term weight regain.10-12
The challenge in treating obesity is access, because Medicare and most private insurance plans do not cover the newly approved treatments. Based on a dated—and arguably discriminatory—perception of weight-loss treatments, and questionable safety and efficacy of older therapies, Congress specifically prohibited Medicare from covering these treatments in 2006 as part of the Medicare Prescription Drug, Improvement, and Modernization Act (MMA). As a result, insurance coverage for weight loss/management is commonly limited to counseling in primary care settings and weight-loss surgery for people with severe obesity. The newer weight-loss therapies have made news primarily as options for those who can afford to pay out of pocket.13
The current status quo—Medicare’s denial of coverage for weight-loss drugs—is a vestige of the days when obesity was considered a lifestyle choice rather than a serious health concern, and Medicare’s associated prohibition on coverage for drugs that were considered cosmetic.14 However, science has since illuminated the complexity of the disease and the many factors that contribute to weight gain, including genetics, social determinants of health, sleep quality and medication usage. An extensive literature has also demonstrated the connection between obesity and many other costly chronic diseases.15-17 Compared to “normal-weight” adults, Americans with obesity experience $2,505 greater annual medical costs, on average.18 Yet Medicare’s coverage policy remains unchanged, and most private insurers have followed its lead.
Bills designed to overturn Medicare’s coverage moratorium have been introduced in each Congress over the last 10 years. The Treat and Reduce Obesity Act (TROA) would expand Medicare Part D coverage to include FDA-approved prescription drugs for chronic weight management. However, high prices for current weight-loss drugs, and rumored for newer drugs, raise concerns about the impact that broad Medicare coverage would have on Part D spending and overall Medicare solvency.
However, focusing solely on the predicted costs of therapies misses or underplays the potential benefits that treating obesity would provide to Medicare overall, and to society at large, through reduced mortality and morbidity. Reducing obesity rates within the Medicare population would decrease not just obesity but also the incidence of many related conditions, such as diabetes and heart disease, that independently raise medical spending and reduce quality of life.15
Our prior research has demonstrated that, even under very conservative assumptions for medication uptake, weight-loss drugs can offer large reductions in medical spending ($140 billion to $188 billion) and net value to society of $1.2 trillion to $1.9 trillion.19 The same model shows that, if all eligible patients were treated with currently approved weight-loss therapies, Medicare would save over $200 billion over 75 years.20 These results hint at the tremendous benefits from broader coverage of weight-loss drugs, but do not account for newer treatments that are both more effective and have fewer side effects.21
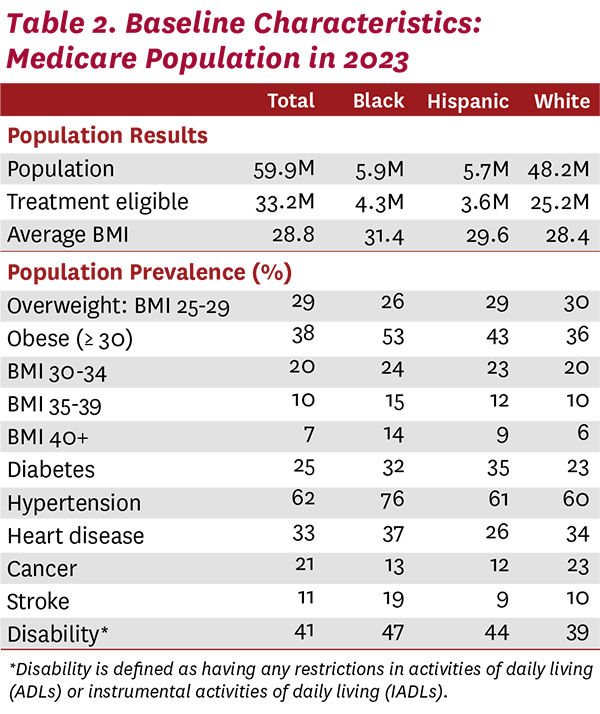
In addition, the disparities in access to treatment cannot be ignored. Obesity disproportionately impacts Black and Hispanic beneficiaries, with 53% and 43% of each population having obesity, respectively. Individuals with lower income and education also face large disparities in obesity rates compared to their higher-income, better-educated counterparts.22
In this paper, we model the potential social benefits—and medical cost offsets—if Medicare provided universal access to this newer class of weight-loss drugs.23 Although we initially modeled a change in Medicare coverage only, such a change would set a precedent for coverage that private insurers may follow. Thus, we also model joint-coverage scenarios and explore the potential benefits to the Medicare population 10, 20 and 30 years into the future.
Methods
Few Americans currently have insurance coverage for drugs to treat obesity. In 2019, the U.S. Government Accountability Office reported that less than 1% of Americans with obesity in 2016 used an FDA-approved drug for weight loss, and the majority of those patients paid for the drug out of their own pocket.9 In this study, we use a well-established microsimulation model to simulate the impacts of new legislation that would provide access to weight-loss therapy for Medicare beneficiaries and possibly other Americans.24-27
We simulate scenarios that show varied insurance coverage for adults 25 years and older using the Future Adult Model (FAM), which simulates lifetime health, medical spending, social services use and economic outcomes using transition probabilities across health and economic states (e.g., incidence of diabetes) in two-year cycles. The model combines data from several large, nationally representative surveys: the Panel Study of Income Dynamics (PSID), the Health and Retirement Study (HRS), the Medical Expenditure Panel Survey (MEPS) and the Medicare Current Beneficiary Survey (MCBS). The primary data source underlying FAM is PSID, a nationally representative longitudinal survey tracking people from age 25 and their family members. Other data sources provide supplemental information on mortality (HRS), quality-adjusted life years (MEPS) and healthcare spending (MEPS, MCBS). As people age, younger populations enter into and refresh the model. We assign health outcomes, social outcomes and risk factors to these populations based on observed trends in the American Community Survey and National Health Interview Survey.
The FAM transition probabilities are modeled as first-order Markov processes, with probabilities based on predicted values from regression models (predictors include age, sex, education, race, health conditions, body mass index and functional status). Health conditions such as heart disease and stroke are measured from answers to PSID questions, and chronic health conditions are treated as absorbing states (that is, once people report being diagnosed with a condition such as diabetes, they are modeled as having that condition until death).
To effectively model long-term weight-management treatments, we made several updates to the obesity-transition component of the model. In each model cycle, FAM estimates an individual’s BMI based on height and weight responses in the PSID. Prior research shows reporting bias in both self-reported height and self-reported weight that can artificially lower estimated BMI.28 Weight management treatments are only indicated for individuals above certain BMI thresholds, so we corrected for these potential biases when selecting the treatment populations. Following prior research, we adjusted for reporting error using data from the National Health and Nutrition Examination Survey, a nationally representative survey that collects data on measured height and weight, to adjust the distribution of self-reported BMI.5 Specifically, we calculated the bias within each quantile of the distribution by estimating the difference between BMI from self-reported heights and weights and BMI from measured heights and weights. We then fit a cubic spline regression to estimate the bias across the whole distribution. We also adjusted the BMI-transition model to account for a longer BMI history for each individual. We now allow an individual’s BMI in the prior three cycles (six years total) to impact the current BMI projections.
Ultimately, FAM projects individual healthcare spending and economic outcomes based on health transitions, functional status, BMI and demographics. For this analysis, we focused on the Medicare population and estimated several economic outcomes, including total Medicare spending and spending for each of Medicare Parts A, B and D. All values are reported in 2023 dollars, with future dollars discounted using a 3% rate. Finally, we also calculated quality-adjusted life-years (QALYs) using predicted EuroQol five-dimensional (EQ-5D) scores based on health states and measures of functional status. Each QALY is then valued at a rate of $150,000 and discounted at 3%. A full description of the model can be found in the technical appendix.
Simulated scenarios for weight loss and treatment coverage
We began our analysis with the U.S. population age 25 and older and simulated their health and economic outcomes over the next 30 years. In each two-year model cycle, individuals exit the model through death and a representative replenishing cohort of 25- to 26-year-old individuals is added. In our status quo scenario, we assumed no change in current coverage policy for weight-management therapies, leaving access to new therapies unchanged and uptake at current levels near zero.9
In the treatment scenarios, we modeled the potential benefits associated with increased access to weight-loss therapies. We modeled a hypothetical treatment that can reduce patients’ BMIa by 20% based on mean weight loss from recent clinical trials in higher-dose regimens.29 Evidence suggests that treatment discontinuation can result in patients rapidly gaining back the weight they had lost. We therefore assumed that patients remain on continuous therapy for the remainder of their lives to maintain their initial weight loss. This assumption is consistent with those made in the 2022 report on the cost-effectiveness of obesity medications published by the Institute for Clinical and Economic Review.30 To reflect natural weight fluctuations after treatment, we modeled weight using our empirically derived BMI transition model, but capped the total weight gain or loss at 5% of post-treatment BMI. We lifted the caps at age 75 to allow for the natural weight loss seen in the elderly population.
In the first treatment scenario, we assumed that Medicare begins covering weight-management therapies in 2023. Given that proposed legislation mandates Medicare coverage only, our baseline simulation assumed that all non-Medicare insurers maintain current coverage levels; privately insured patients’ access to and uptake of weight-management therapies is unchanged.
As noted, passing TROA may result in broader coverage for weight-loss therapies for patients with private insurance as well. We modeled this in a second treatment scenario, where all patients 25 and older covered by either Medicare or private insurance gained access to weight-loss therapies starting in 2023.
In both treatment scenarios, we defined treatment eligibility as BMI ≥ 30 kg/m2, or BMI ≥ 27 kg/m2 in the presence of at least one weight-related comorbidity (hypertension or type 2 diabetes). We assume immediate, 100% uptake of the treatment among eligible patients and zero discontinuation. Although aspirational, these assumptions allowed us to capture the total potential benefits from a policy that provides widespread access to weight-loss therapies. Put another way, our estimates can help us better understand the opportunity cost of failing to treat a disease for which there is an effective treatment.
Alternative scenarios addressing uncertainty
Studies on long-term treatment with new weight-loss drugs in real-world populations are ongoing, so there is uncertainty about the size and durability of the initial weight loss following treatment. To address these concerns, we ran several additional scenarios to test key treatment assumptions. The pivotal clinical trial for one newer weight-loss drug, tirzepatide (brand name Mounjaro), tested three different dosing strengths, and our baseline scenario assumption of a 20% weight reduction is consistent with the results for the higher doses. However, the lowest-dose regimen resulted in a 15% weight reduction, so we also modeled a 15% weight-loss treatment effect.29
We also modeled a scenario incorporating a less optimistic outcome for weight maintenance. Instead of assuming that ongoing treatment would allow patients to maintain their initial weight loss, we modeled the alternative assumption that they return to their pretreatment weight trajectories. In other words, although patients remain on treatment, their now-lower weight continues to increase according to its pretreatment trajectory, without any cap or floor. Our BMI-transition model, which includes six years of lagged BMI data, predicts each patient’s future weight. A summary of all scenarios modeled can be found in Table 1.
Results
Our model of Americans ages 25 and older in 2023 included a starting population of 68.4 million beneficiaries on Medicare. Table 2 shows the baseline characteristics for the Medicare population, with almost 40% of the beneficiaries having obesity in 2023. Hypertension was the most prevalent obesity-related disease, impacting 62% of beneficiaries, followed by heart disease (33%) and diabetes (25%). With higher rates of obesity and related comorbidities, Black and Hispanic beneficiaries will see the greatest benefits from broad access to weight-loss treatments. In fact, over half of the Black Medicare population has obesity, and three-fourths suffer from hypertension. Although the obesity rate is slightly lower for the Hispanic population, Hispanics had the highest rate of diabetes (35%). These baseline statistics highlight the fact that current behavioral interventions for weight loss have proven less effective in minority populations and have expanded existing health disparities.31-33 They also highlight that passage of legislation to allow coverage of FDA-approved treatments for obesity is just the start of the process—diagnosis, treatment and patient adherence to a prescribed treatment regimen are also necessary.
Scenario results
Our simulation results suggest that if all people eligible for treatment were provided access and treated, Medicare would benefit from large cost offsets, or savings, and quality-of-life gains to patients. Our analysis quantifies benefits and medical cost offsets, but does not account for the costs of the treatment itself. We view the latter as a question of how manufacturers and government will share the social benefits between them.
Relative to the status quo, the results in Table 3 suggest that the first 10 years of broad coverage will produce $176 billion in cost offsets to Medicare and over $700 billion in cost offsets after 30 years. These savings represent a reduction in healthcare spending from fewer hospitalizations, surgeries, doctors’ visits, drugs, nursing home stays and other medical needs associated with a healthier Medicare population. If private insurers were to also cover weight-loss drugs, the average 65-year-old entering Medicare would be healthier, with a lower BMI than under either the status quo scenario or the Medicare-only coverage scenario. Entering beneficiaries would be less likely to have costly comorbid diseases like hypertension, diabetes or heart disease, creating additional cost offsets for Medicare. The results suggest that, after 30 years of coverage for both Medicare and private insurance patients, Medicare could save almost $1.5 trillion.
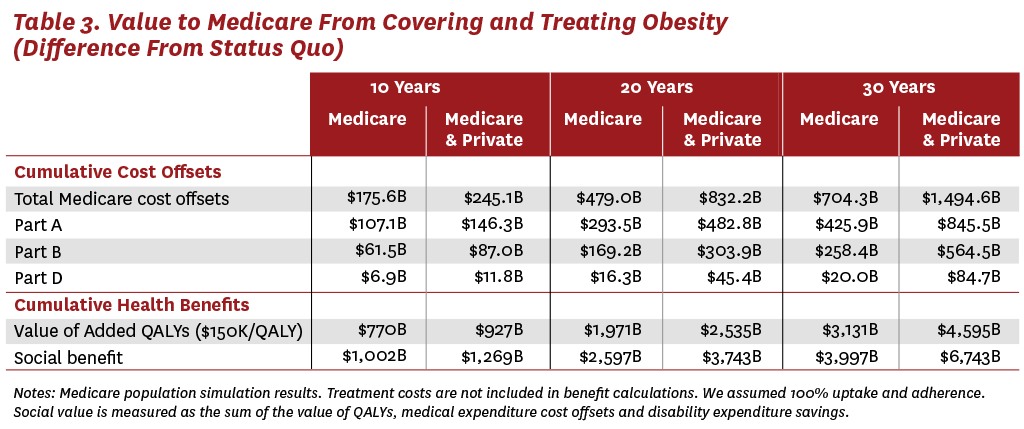
The majority of the projected cost offsets to Medicare (60%) occur in Medicare Part A (hospital inpatient care) spending, with the rest coming from savings to Medicare Parts B (outpatient care) and D (drug benefits). In fact, we estimate that Medicare Part A spending will fall by $846 billion after 30 years of Medicare and private insurance coverage for weight-loss therapies. These savings will be essential, as the 2023 Medicare Trustee’s Report estimates that Medicare’s Hospital Insurance (HI) Trust Fund, which finances Part A spending, will become insolvent in 2031. The HI Trust Fund is financed through payroll taxes, and spending is predicted to outpace revenues in the coming years, which will deplete any surplus revenues. Insolvency would mean that Medicare might be unable to reimburse hospitals and skilled-nursing facilities for their services. By contrast, Medicare Parts B and D are financed through general federal revenues and do not face the same insolvency risks. Thus, treating obesity with weight-loss drugs has the potential to generate much-needed cost offsets to Medicare Part A, while any spending associated with the drugs will come from Part D.
In addition to reducing healthcare costs, treating obesity is also expected to improve quality of life for patients. If each QALY is valued at $150,000, we estimate that Medicare coverage for weight-loss drugs will generate $770 billion in health and longevity improvements for the Medicare population over the next 10 years. Like the reductions in medical spending, the quality-of-life benefits increase with longer time horizons and with the addition of private insurance coverage. After 30 years, we estimate that Medicare and private insurance coverage for weight-loss therapies will generate almost $4.6 trillion in quality-of-life benefits.
The simulation results also demonstrate that treating obesity will reduce the incidence of many related diseases in the Medicare population. As shown in Table 4, covering weight-loss therapies is expected to reduce average BMI in the Medicare population over time. After just 10 years of Medicare coverage, we estimate that the average BMI in Medicare will fall 3.1 points compared to the status quo. Over a longer horizon, and with the addition of private insurance coverage, the average BMI among the Medicare population could fall by more than 4 points. The model also predicts that the incidence of all related comorbidities could fall dramatically in the Medicare population. For example, after 30 years of Medicare coverage for weight-loss therapies, the prevalence of diabetes is expected to fall by 7.7%, and could fall by 24% if private insurers also provide coverage. Treating obesity with weight-loss therapies could also lead to reductions in the prevalence of hypertension, heart disease, cancer, lung disease, stroke and disability. The benefits of providing access to these therapies increases over longer time horizons and with broader coverage.
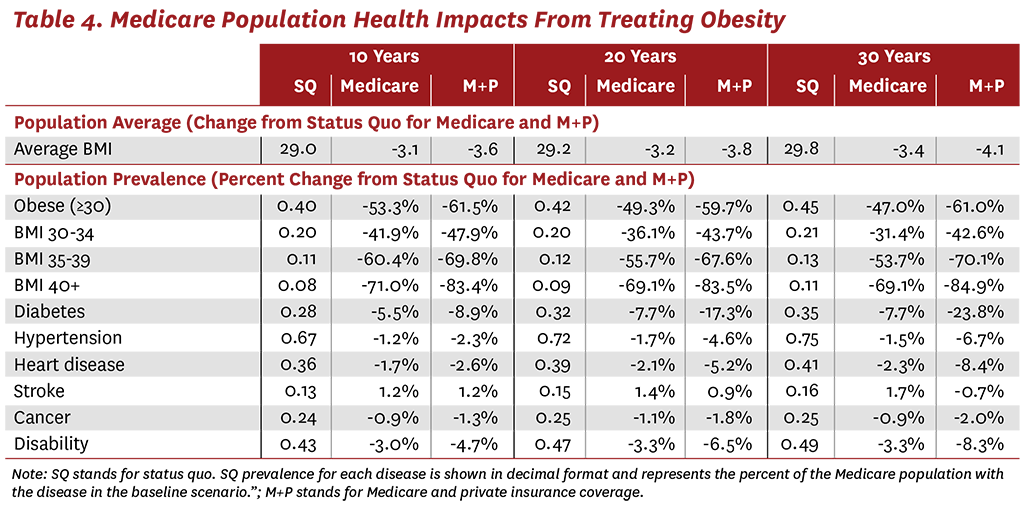
Although we estimate clear benefits from treating just the Medicare population, our results also highlight the importance of treating obesity at younger ages, before patients develop chronic conditions such as diabetes and heart disease. Thus, the greatest reductions in chronic diseases are seen when both private insurance and Medicare cover weight-loss treatments. After 30 years of coverage, the prevalence of heart disease and disability could each fall by more than 8% in the Medicare population. These reductions are even more impressive when one considers that weight loss in the population with obesity increases the average life expectancy, and beneficiaries therefore have more time to develop comorbid diseases. In other words, as life expectancy increases, the odds of developing cancer, stroke, hypertension and other diseases also naturally increase. Despite this dynamic, treating obesity with current drugs is estimated to have significant health benefits.
Alternative scenarios
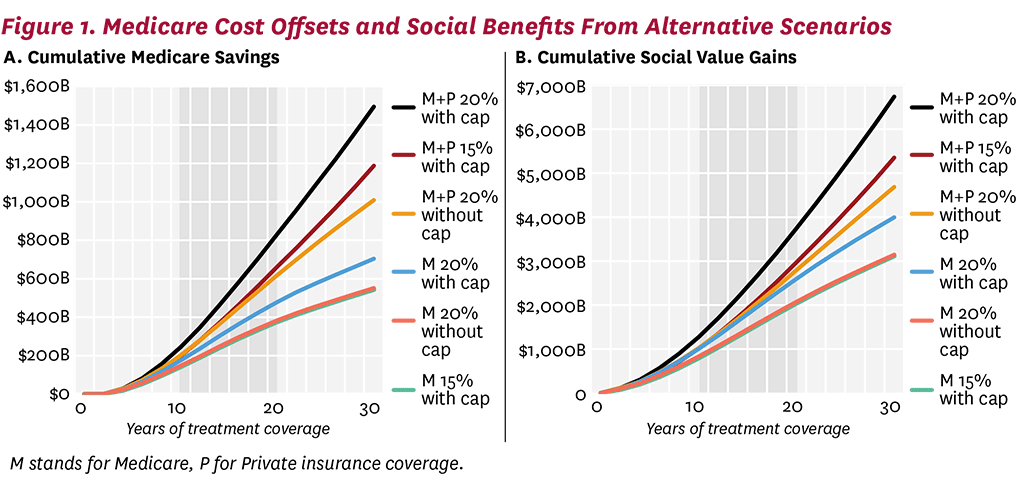
The long-term efficacy of innovative weight-loss drugs is unknown, but the benefits of widespread coverage is clear even under relatively conservative efficacy assumptions. Figure 1A shows the cumulative cost offsets to Medicare under the alternative scenarios modeled. Even without a cap on how much weight can be regained, or limiting the initial BMI reduction to 15%, we see substantial cost offsets. After 30 years of Medicare coverage, each of our modeled scenarios generates over $500 billion in cumulative offsets for Medicare. If private insurance also covers weight-loss drugs along with Medicare, cumulative savings to Medicare increase to more than $1 trillion (note that this excludes any savings that would accrue to private insurers directly). The same pattern is seen in the cumulative social benefits (quality-of-life benefits minus medical spending accrued through longer life span, not including the cost of treatment and disability payments), generated by each treatment scenario (Figure 1B). Thirty years of Medicare coverage for therapies that achieve and maintain a 15% weight reduction will generate $3 trillion in benefits to society, which is very similar to the benefits generated by scenarios that allow future weight gain. Again, the potential benefits of these therapies in the Medicare population is almost doubled by the addition of private coverage. Even conservative assumptions generate large social benefit estimates.
Value of treating obesity in subpopulations
As Congress considers the costs and benefits of Medicare coverage for weight-loss treatments, a natural compromise may be to limit coverage to subpopulations of patients who stand to benefit the most from treatment. One potential approach would be to offer treatment only to patients with a BMI above a certain threshold. To explore the potential impact of such policies, we estimated the average social benefits per person per treatment year for patients in different age, BMI and income groups. We simulated a representative cohort of the adult U.S. population that would qualify for treatment under scenarios with and without lifetime weight-loss treatment. We measured the social benefits from treatment as the difference in QALYs, medical expenditures and disability expenditures compared to no treatment. We then divided the total social benefits by treatment years to estimate an average benefit per year of treatment. It is important to note that our calculation of treatment years is based on very conservative assumptions. In the model, patients remain on treatment from the time of initiation until their death. In reality, some patients will discontinue treatment and still receive a large portion of the health benefits from the initial 20% BMI reduction. This would be especially true in older populations that, on average, lose weight naturally as they age. In this way, our estimates of the annual value from treatment can be seen as a lower threshold. Figure 2A graphs the average social benefit per treated year by age of treatment initiation, which demonstrates that treatment at younger ages generates the greatest social benefit. This result reflects the fact that younger adults will accrue benefits from reduced obesity over a longer time period compared to older adults. They are also less likely to have diabetes, heart disease and other chronic conditions related to obesity. Thus, treating patients earlier in life may prevent development of these expensive diseases that increase mortality and reduce quality of life.
Figure 2B breaks out the previous comparison of average social benefit per treated year by treatment age into different pre-treatment BMI categories. The results show that the average annual social benefit from treatment is greatest for patients in moderate BMI categories (BMI between 30 and 40). In other words, there is no value-based rationale for restricting access to the lowest or highest BMI populations.
As the baseline summary statistics in Table 1 demonstrate, obesity disproportionately impacts minority populations and populations with lower socioeconomic status. The literature has explored cultural, behavioral, environmental and economic reasons for these disparities with little consensus on the primary drivers. Regardless of the root causes, the statistics suggest that minority populations may benefit the most from insurance coverage for weight-loss treatments. In Figures 2C and 2D, we graph the average social benefits of weight-loss treatment per treated year by age categories split by race and education, respectively. We find that treating Black and Hispanic adults generates greater social benefits compared to their white counterparts across almost all age categories. The same is true for the non-college-educated U.S. population—their treatment generates more social benefit than treating those with a college education in all age categories. These results suggest that providing broad coverage for weight-loss treatment would help narrow current health disparities.
Discussion
Since TROA’s introduction in Congress 10 years ago, several important developments have occurred. Obesity in the U.S. has continued to outpace forecasts and innovative new treatments have emerged that reduce body weight effectively and safely. This makes TROA both more important and potentially more expensive than ever.
Our analysis demonstrates that treating obesity at current efficacy rates generates substantial social benefits. The cumulative social benefits generated from Medicare-only coverage for new obesity treatments over the next 10 years is estimated at almost $1 trillion, or roughly $100 billion per year. If private insurance also covers weight-loss drugs, the benefits will rise further.
We have also shown that TROA would generate significant cost offsets for Medicare. In the first 10 years alone, covering weight-loss therapies would save Medicare $175 billion to $245 billion, depending on whether private insurance follows suit. Over 60% of these savings would accrue to Medicare Part A by reducing hospital inpatient care demands and demand for skilled nursing care. Such savings would be very timely, given estimates that Medicare Part A will become insolvent in the coming decade.
The social benefits of treatment increase over time, as do the savings to Medicare. After 30 years of covering weight-loss drugs, Medicare Part A could save from $436 billion to $846 billion. As Medicare and pharmaceutical manufacturers negotiate how they will divide the surplus, it is important to understand that spending for weight-loss drugs will come from Part D, which, unlike Part A, is financed through general federal revenues. In other words, most of the Medicare savings from treating obesity will accrue to Part A, while the drug costs will be financed by Part D.
Passing TROA could also improve health equity within the Medicare community. Obesity disproportionately impacts Black and Hispanic beneficiaries. Individuals with lower income and education also face higher obesity rates than their higher-income, better-educated counterparts.22 Research shows that health breakthroughs in medical technologies that simplify healthcare and reduce patient effort can reduce these health disparities.34 For example, prior to the discovery of beta blockers, diet, exercise and other behavioral self-management methods were the leading treatment options for hypertension.
Following the initial FDA approval for beta blockers in 1976, we saw reductions in both hypertension and cardiac disease that were equal across income levels.34 In the same way that beta blockers simplified the treatment of hypertension, new weight-loss therapies can simplify the treatment of obesity. Research also shows that Black adults experience less weight loss from behavioral interventions than their white counterparts, and that individuals with more limited financial resources have less access to behavioral weight-loss interventions.31-33 This means that the status quo (i.e., limited access to weight-loss treatments) will actually widen existing disparities in obesity and related comorbid diseases like diabetes, hypertension, heart disease, lung disease, cancer and stroke.
If society is better off through the use of these medications, the question naturally arises: What is a fair price to pay for universal access? In one analysis of 200 drugs, innovators (manufacturers) captured 13%, on average, of the value generated by the drugs they produced, with the remaining surplus going to consumers.35 In the case of weight-loss treatments under Medicare coverage, 13% would amount to roughly $13 billion in revenues per year going to manufacturers, with the remaining $87 billion per year going to consumers. In other words, if the average 13% division were applied here, manufacturers’ revenues from new obesity treatments in the Medicare population alone would exceed the revenues for top-selling drugs in the U.S. in 2023.
Perhaps a better comparison is statins. Cardiovascular disease was the leading cause of mortality in the U.S. 50 years ago. The development of statins in the late 1980s is credited with huge reductions in cardiovascular disease. Over 20 years, it is estimated that statins created $1.252 trillion in social value, of which manufacturers captured 24%.36
While the benefits of obesity treatments are large, so are the costs. Further, enormous heterogeneity exists in patient benefits. We have shown that younger patients with a BMI between 30 and 40 are likely to experience the greatest annual value from treatment. This makes obesity treatment ripe for some outcomes-based pricing approaches that would reward the innovators but also encourage speedier uptake. In 2018, we proposed a three-part pricing solution in the context of PCSK9 inhibitors; that model is applicable here as well.23 Future work will examine such pricing models.
The medical community has been sounding the alarm about rising obesity rates for years. Congress should seriously consider the socioeconomic benefits of lifting the moratorium on Medicare coverage for weight-loss drugs, enabling Medicare to work with manufacturers to create reimbursement solutions that provide broad access to new treatments. Such a policy change would generate huge value to society and narrow existing racial and economic health disparities.
Footnotes
a. BMI is measured as weight in kilograms divided by height in meters squared. Thus, a 20% reduction in weight for and individual is equal to a 20% reduction in BMI.
Disclosure:
The Schaeffer Center White Paper Series is published by the Leonard D. Schaeffer Center for Health Policy & Economics at the University of Southern California. Papers published in this series undergo a rigorous peer review process, led by the Director of Quality Assurance at the USC Schaeffer Center. This process includes external review by at least two scholars not affiliated with the Center. This white paper was supported by the Schaeffer Center, which receives funding from foundations, government agencies, individuals, and corporations — including Eli Lilly & Company and other companies that may have interests in obesity treatments. The Future Adult Model was developed with support from the National Institute on Aging (P30AG024968). A complete list of sponsors can be found in our annual report (available here). At all times, the independence and integrity of the research is paramount and the Center retains the right to publish all findings from its research activities. The views expressed herein are those of the authors and do not necessarily represent the views of the Schaeffer Center or its sponsors. Disclosures reported by authors are available here.
References
- Tartof, S. Y., L. Qian, V. Hong et al. (2020). Obesity and Mortality Among Patients Diagnosed With COVID-19: Results From an Integrated Health Care Organization. Annals of Internal Medicine, 173 (10): 773-81.
- Flegal, K. M., B. K. Kit, H. Orpana and B. I. Graubard. (2013). Association of All-Cause Mortality With Overweight and Obesity Using Standard Body Mass Index Categories: A Systematic Review and Meta-Analysis. JAMA, 309 (1): 71-82.
- Lakdawalla, D. N., J. Bhattacharya and D. P. Goldman. (2004). Are the Young Becoming More Disabled? Health Affairs, 23 (1): 168-76.
- Goldman, D., P-C. Michaud, D. Lakdawalla, Y. Zheng, A. Gailey and I. Vaynman. (2010). The Fiscal Consequences of Trends in Population Health. National Tax Journal, 63 (2): 307-30.
- Ward, Z. J., S. N. Bleich, A. L. Cradock et al. (2019). Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. New England Journal of Medicine, 381 (25): 2440-50.
- Pollack, A. (2013). A.M.A. Recognizes Obesity as a Disease. New York Times, June 18.
- Hoagland, G. W., A. Parekh, G. St. John and M. Lovegrove. (2022). Expanding Access to Obesity Treatments for Older Adults. Bipartisan Policy Center, February 9.
- Saxon, D. R., S. J. Iwamoto, C. J. Mettenbrink et al. (2019). Antiobesity Medication Use in 2.2 Million Adults Across Eight Large Health Care Organizations: 2009-2015. Obesity, 27 (12): 1975-81.
- U.S. Government Accountability Office. (2019). Obesity Drugs: Few Adults Used Prescription Drugs for Weight Loss and Insurance Coverage Varied. August 9.
- Gasoyan, H., G. Tajeu, M. T. Halpern and D. B. Sarwer. (2019). Reasons for Underutilization of Bariatric Surgery: The Role of Insurance Benefit Design. Surgery for Obesity and Related Diseases, 15 (1): 146-51.
- Lupoli, R., E. Lembo, G. Saldalamacchia, C. K. Avola, L. Angrisani and B. Capaldo. (2017). Bariatric Surgery and Long-Term Nutritional Issues. World Journal of Diabetes, 8 (11): 464-74.
- Adams, T. D., L. E. Davidson, S. E. Litwin et al. (2012). Health Benefits of Gastric Bypass Surgery After 6 Years. JAMA, 308 (11): 1122-31.
- Robledo, A. (2023). These Celebrities Have Spoken Out About Ozempic and Other Drugs Used for Weight Loss, Including a Few Who Said They’ve Taken Them. BuzzFeed, January 31.
- Health Policy Alternatives. (2003). Prescription Drug Coverage for Medicare Beneficiaries: A Summary of the Medicare Prescription Drug, Improvement, and Modernization Act of 2003. Henry J. Kaiser Family Foundation, December 10.
- Guh, D. P., W. Zhang, N. Bansback, Z. Amarsi, C. L. Birmingham and A. H. Anis. (2009). The Incidence of Co-morbidities Related to Obesity and Overweight: A Systematic Review and Meta-Analysis. BMC Public Health, 9: 1-20.
- Bogers, R. P., W. J. Bemelmans, R. T. Hoogenvee et al. (2007). Association of Overweight With Increased Risk of Coronary Heart Disease Partly Independent of Blood Pressure and Cholesterol Levels: A Meta-Analysis of 21 Cohort Studies Including More Than 300,000 Persons. Archives of Internal Medicine, 167 (16): 1720-28.
- Bhaskaran, K., I. Douglas, H. Forbes, I. dos-Santos-Silva, D. A. Leon and L. Smeeth. (2014). Body-Mass Index and Risk of 22 Specific Cancers: A Population-Based Cohort Study of 5.24 Million UK Adults. The Lancet, 384 (9945): 755-65.
- Cawley, J., A. Biener, C. Meyerhoefer et al. (2021). Direct Medical Costs of Obesity in the United States and the Most Populous States. Journal of Managed Care and Specialty Pharmacy, 27 (3): 354-66.
- Kabiri, M., A. Sexton Ward, A. Ramasamy et al. (2020). The Societal Value of Broader Access to Antiobesity Medications. Obesity, 28 (2): 429-36.
- Kabiri, M., A. Sexton Ward, A. Ramasamy et al. (2021). Simulating the Fiscal Impact of Anti-obesity Medications as an Obesity Reduction Strategy. Inquiry: The Journal of Health Care Organization, Provision, and Financing, 58.
- Waidmann, T. A., E. Waxman, V. Pancini, P. Gupta and L. P. Tabb. (2022). Obesity Across America: Geographic Variation in Disease Prevalence and Treatment Options. Urban Institute, February 17.
- Fouad, M. N., K. J. Waugaman and G. R. Dutton. (2022). The Complex Contributors to Obesity-Related Health Disparities: Introduction to the Special Issue. American Journal of Preventive Medicine, 63 (1): S1-5.
- Goldman, D. P., K. Van Nuys, W-H. Cheng et al. (2018). A New Model for Pricing Drugs of Uncertain Efficacy. NEJM Catalyst, 4 (6).
- Richardson, A. S., R. Zutshi, P. Nguyen, B. Tysinger and R. Sturm. (2022). Microsimulation Projections of Obesity Interventions on Cardiometabolic Health Disparities in the United States. Obesity, 30 (1): 62-74.
- Chaturvedi, R., T. Gracner, B. Tysinger, K. Narain, D. Goldman and R. Sturm. (2022). The Long-Term Value of Bariatric Surgery Interventions for American Adults With Type 2 Diabetes Mellitus. Annals of Surgery, 10.1097.
- Seabury, S. A., S. Axeen, G. Pauley et al. (2019). Measuring the Lifetime Costs of Serious Mental Illness and the Mitigating Effects of Educational Attainment. Health Affairs, 38 (4): 652-59.
- Reif, J., H. Heun-Johnson, B. Tysinger and D. Lakdawalla. (2021). Measuring the COVID-19 Mortality Burden in the United States: A Microsimulation Study. Annals of Internal Medicine, 174 (12): 1700-9.
- Ward, Z. J., M. W. Long, S. C. Resch et al. (2016). Redrawing the U.S. Obesity Landscape: Bias-Corrected Estimates of State-Specific Adult Obesity Prevalence. PLOS One, 11 (3): e0150735.
- Jastreboff, A. M., L. J. Aronne, N. N. Ahmad et al. (2022). Tirzepatide Once Weekly for the Treatment of Obesity. , 387 (3): 205-16.
- Atlas, S. J., K. Kim, M. Beinfeld, V. Lancaster, E. Nhan, P. W. Lien, K. Shah, D. R. Touchette, A. Moradi, D. M. Rind, S. D. Pearson and F. L. Beaudoin. (2022). Medications for Obesity Management: Effectiveness and Value. Institute for Clinical and Economic Review, August 31.
- Davis, K. K., D. F. Tate, W. Lang et al. (2015). Racial Differences in Weight Loss Among Adults in a Behavioral Weight Loss Intervention: Role of Diet and Physical Activity. Journal of Physical Activity and Health, 12 (12): 1558-66.
- Lewis, K. H., S. A. Edwards-Hampton and J. D. Ard. (2016). Disparities in Treatment Uptake and Outcomes of Patients With Obesity in the USA. Current Obesity Reports, 5: 282-90.
- Wingo, B., T. Carson and J. Ard. (2014). Differences in Weight Loss and Health Outcomes Among African Americans and Whites in Multicentre Trials. Obesity Reviews, 15: 46-61.
- Goldman, D. P., and D. N. Lakdawalla. (2005). A Theory of Health Disparities and Medical Technology. Contributions in Economic Analysis & Policy, 4 (1): 1-30.
- Philipson, T., and A. Jena. (2006). Surplus Appropriation From R&D and Health Care Technology Assessment Procedures. National Bureau of Economic Research, Working Paper 12016.
- Grabowski, D. C., D. N. Lakdawalla, D. P. Goldman et al. (2012). The Large Social Value Resulting From Use of Statins Warrants Steps to Improve Adherence and Broaden Treatment. Health Affairs, 31 (10): 2276-85.

You must be logged in to post a comment.