Key Takeaways
- Many U.S. patients with serious illnesses do not receive palliative care to manage their pain and other symptoms through patient-centered care coordination and planning.
- Greater use of palliative care could potentially increase the value of U.S. healthcare spending, but public and private payers lack the evidence base needed to validate increased access to palliative care across settings and patient populations.
- Developing a research agenda on the economic issues related to palliative care is a critical first step in identifying effective components of palliative care and how to efficiently provide these services to those most likely to benefit.
Abstract
Current research assessing the economic outcomes of palliative care is limited, and policymakers, payers, patients and clinicians need more information to appropriately and systematically implement palliative care in the United States. Filling this research gap can help identify optimal ways to deliver and pay for palliative care across settings as well as strategies to encourage palliative care referrals and uptake. Moreover, projecting future needs for palliative care through modeling and other analytic techniques can inform care provision, future research and, ultimately, policy decisions.
In 2020, the USC Schaeffer Center for Health Policy & Economics established an advisory panel to identify and consider how to address the gaps in research at the interface of economics and palliative care. The panel includes clinicians, economists and select participants from health system, payer and policy domains. This background paper, authored by the panel chairs, aims to set the stage for developing a consensus-based research agenda that advances palliative care in the United States and makes a case for funders to support this research.
A palliative care research agenda can help lay the foundation for building a strong evidence base to guide public policies meant to advance affordable, equitable, high-quality, patient-centered care. Along with providing an overview of palliative care, this paper examines the:
- Growing need for palliative care and increases in availability of palliative care services, especially in light of the COVID-19 pandemic and related health disparities and inequities
- Identification of barriers to palliative care
- Systematic reviews of palliative care related to health outcomes
- Economic literature related to palliative care
- Future demand for palliative care, including use of microsimulation models to project demand and related workforce issues
Overview of Palliative Care
Derived from the Latin term pallium for a cloak, the modern term palliate—while never literally referring to a garment—took on the figurative meaning of a “cloak” of protection, especially in lessening the intensity of serious disease.1 Today, palliative care refers to a range of services aimed at improving the quality of life of seriously ill patients, as well as their families. To achieve this aim, palliative care “attends to the physical, functional, psychological, practical, and spiritual consequences of a serious illness,”[2] ideally through interdisciplinary team-based care. As such, palliative care is disease-agnostic and takes a holistic approach to pain and other symptom management, care coordination and planning, and assessment and support of caregiver needs.[3,4]
With origins in the hospice movement5 and obvious benefits for patients lacking curative options, palliative care is often conflated with hospice or end-of-life care.[6] Over time, the meaning of palliative care has evolved, however, to include a broad range of services to improve the quality of life of both patients and caregivers facing any serious illness—not just at the end of life.[7] The National Consensus Project (NCP) for Quality Palliative Care guidelines make clear that palliative care is appropriate at any stage in a serious illness and can be integrated with curative care.
Palliative care can be given in any setting, including but not limited to hospitals, physician offices, long-term-care facilities, cancer centers and homes. While hospice care, which almost always includes palliative care, also can be provided across settings, it specifically focuses on the quality of life of patients with an advanced illness near the end of life, defined by U.S. payers such as Medicare to mean life expectancy of six months or less.
Two characteristics are central to palliative care. First, as described by the NCP, palliative care involves understanding the goals and preferences of patients, their families and caregivers. These goals and preferences directly inform the care plan. Second, palliative care by definition is interdisciplinary. To attend to the physical, emotional and spiritual needs of patients, their families and caregivers, palliative care, according to NCP guidelines, should be provided by a team of “physicians, advanced practice registered nurses, physician assistants, nurses, social workers, chaplains, and others based on need.” That said, the consensus guidelines also envision any clinician treating patients with serious illness adopting palliative care “principles and practices” and consulting with and/or handing care off to specialized palliative care teams in the most complex cases.
Growing Need and Increasing Palliative Care Availability
As the population ages, the number of older people with multiple chronic conditions that can lead to serious illnesses is growing. In the United States, between 2016 and 2060, the number of people aged 65 and older will grow from 49 million—15% of the population—to about 98 million, or nearly 25% of U.S. residents.8 The prevalence of heart disease, diabetes, arthritis, Alzheimer’s disease and other dementias, and cancer—major drivers of illness, disability, deaths and healthcare costs—all increase with aging.
Over the past two decades, the United States has seen tremendous growth in palliative care capabilities. As hospice care became a more mainstream part of care delivery, payers, providers and policymakers began to realize the potential benefits of palliative care for patients with serious illness at any stage—namely improved quality of life and reduced costs.[9]
Palliative medicine is an official subspecialty of the American Board of Medical Specialties sponsored by Internal Medicine, Family Medicine and eight additional parent boards as of 2008,10 and the numbers of specialists and programs have grown considerably in the past several decades. Similarly, from 2000 to 2016, the percentage of U.S. hospitals with 50 or more beds with a palliative care program tripled (see Figure 1).[11] The National Academy of Medicine (formerly the Institute of Medicine) has outlined several factors contributing to this growth—increases in the numbers and needs of the elderly, recognition of the important role of caregivers, increased prevalence of chronic diseases and public attention to assisted suicide, as well as a growing body of research pointing to patient satisfaction, improved quality of life and reduced costs.[12] Despite this growth, many seriously ill people in the United States, as well as in developed countries with more integrated healthcare systems, either do not receive palliative care or receive it very late in the disease trajectory.[13]
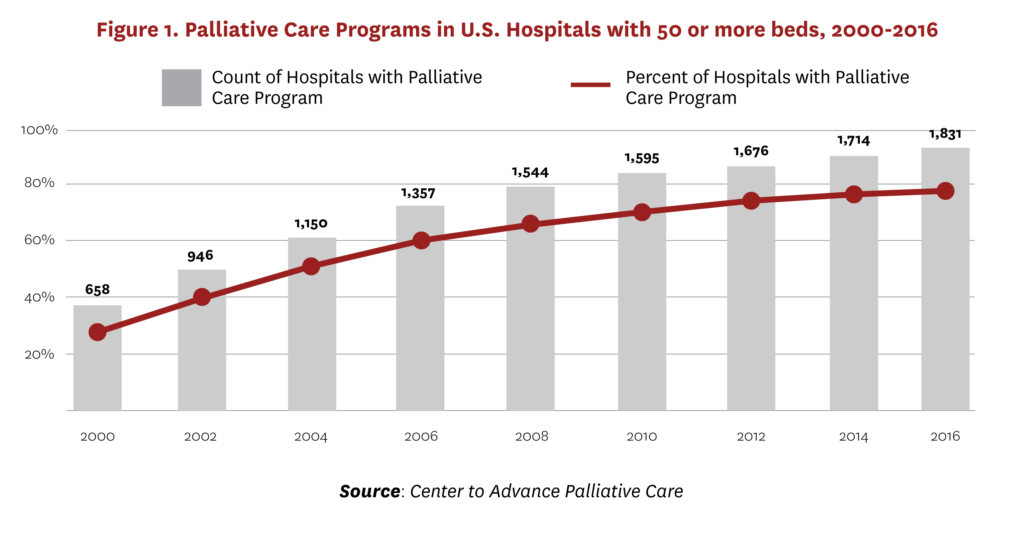
Palliative Care and the Pandemic
As the United States responds to the worst public health crisis in a century with more than 600,000 people dead from COVID-19 and millions at risk for serious illness with limited treatments, palliative care is increasingly in demand.[14] People who are elderly, frail, and/or have underlying chronic or serious illness—palliative care’s main patient population—are at highest risk of serious illness if they contract the coronavirus.[15] The core principles of palliative care—managing pain and symptoms, coordinating care, encouraging advance care planning, and supporting family and caregiver needs—are critical touchstones for health professionals on the frontlines of the pandemic.
Palliative care specialists are urging all clinicians to consider symptom management and skilled communication with patients and families as “essential clinical services” during the pandemic.[16] At the same time, the sheer scale of the pandemic threatened to overwhelm the healthcare system and palliative care teams, raising questions, for example, about how best to practice palliative care when family members can’t visit loved ones in the hospital, difficult decisions must be made via video calls, and patient and family preferences can collide with allocation of scarce resources.[17]
Moreover, the pandemic has underscored and highlighted the significant inequities facing members of racial and ethnic minority groups in the United States, both generally in the larger societal context and specifically within healthcare. By almost any economic or health measure, communities of color have disproportionately borne the burdens of the pandemic.[18]
Overcoming Barriers
“Honest discussion about serious illness is the hallmark of exceptional medical care. We should all be doing this, every day, but even in the best of circumstances there are barriers. No one wants to talk about death or unpleasant things. No one wants to be seen as taking away hope. It’s not uncommon for a doctor to tell me, ‘My patient is not ready to hear this yet,’ even when all the clinicians involved in the case are clear about a bleak outcome.”
— Jessica L. Israel, MD, senior vice president of geriatrics and palliative care at RWJBarnabas Health, June 24, 2020, Harvard Business Review
Barriers to Palliative Care
Despite increased awareness and growth, structural barriers such as “limited palliative care training opportunities and workforce and inappropriate funding systems” likely have limited the growth of palliative care in the United States.19 The move away from strictly fee-for-service payments toward value-based payment design in the U.S. may lessen these barriers, a point discussed later in this paper.
Moreover, many seriously ill patients who could benefit from palliative care never receive consultations. For example, across conditions ranging from cardiovascular disease to congestive heart failure to cancer, typically few patients receive referrals to specialist palliative care.20, 21
Barriers to palliative care referrals include:
- Confusion about what palliative care is
- Lack of awareness of palliative care resources
- Clinician reluctance to refer patients
- Reluctance of patient and/or family to be referred
- Restrictive palliative care program eligibility criteria—particularly under the U.S. hospice benefit and in some European countries22
Larger systemwide issues, including fragmented care delivery and perverse payment incentives, also pose barriers to greater referrals and uptake of palliative care by clinicians and patients, respectively, in the United States.
Fragmented care delivery. Closer consideration of the incentives within the U.S. healthcare system and which parties stand to benefit from the value creation sheds light on some key—albeit not the only—barriers to widespread adoption. Care is often fragmented—spread across numerous specialized providers and sometimes health systems—making care coordination challenging.[23] While palliative care aims to coordinate care, the handoff to/trigger for such care is more easily missed in a highly decentralized system.
Perverse payment incentives. Despite continued movement toward risk-based payment models, most U.S. healthcare is still reimbursed through fee-for-service payment that rewards procedures and diagnostic testing more generously than care management.[24] To the extent that palliative care substitutes for curative treatment, fee-for-service payment means health systems can be hurt financially by shifting patients away from procedures toward time-intensive care. Thus, even in circumstances in which palliative care may increase value to the system as a whole, providers may not share in these gains. At a more prosaic level, the fee-for-service payment system, with fees attached to codes for different types of care, is better set up to reimburse for procedures and tests than what can be the complex, time-consuming processes inherent to palliative care.[25]
Medicare, which covers approximately 80% of people who die annually in the United States,[26, 27] has made considerable progress toward value-based payments. This move, which includes growth in Medicare Advantage (MA) and in risk-sharing models within traditional Medicare, should provide better incentives for palliative care.
Over the past 15 years, MA—which pays private insurers a risk-adjusted fee to administer bundled inpatient, outpatient and typically prescription drug benefits to Medicare beneficiaries who opt into these plans—has grown from about 13% of beneficiaries in 2005 to over a third of beneficiaries today.[28] In the case of Kaiser Permanente, the largest nonprofit MA plan, the plan and the provider are effectively (if not legally) the same entity. Similarly, in many, but not all other cases, insurers use a “delegated model” where they contract with providers to take financial risk for their patients’ care. These providers are paid a per-member monthly fee to manage the care of MA enrollees and thus stand to gain both financially and in terms of quality ratings from appropriate use of palliative care.
Medicare’s hospice benefit, which was implemented in 1982, currently interacts with MA in a way that promotes access to hospice care.[29, 30] Specifically, since hospice is paid as a separate benefit, once an MA enrollee enters hospice, the MA plan is no longer responsible for the expenses incurred by these high-cost individuals. Instead, costs are borne by original Medicare under the hospice benefit. Because MA data have only recently become available to researchers, e.g., through MA encounter data,31 we know relatively little about the use of palliative care across MA plans and/or how hospice use compares in MA plans relative to fee-for-service Medicare, where financial risk is not shared with providers. The Centers for Medicare & Medicaid Services plans to launch a demonstration program in 2021 to test the impact of “carving in,” or including, hospice within the MA plan benefit on cost and quality of care as well as care coordination and access to palliative care outside Medicare’s traditional hospice benefit. The demonstration may alter practice patterns regarding access to both hospice and palliative care.
Medicare also has moved toward value-based care that is more consistent with palliative care through the growth of accountable care organizations (ACOs) and other special payment models in original Medicare. Although largely built on the existing fee-for-service Medicare payment system, these models provide incentives for coordinated care by investing in data infrastructure and offering providers a share in any savings (and increasingly in any loss) to Medicare relative to projected targets and subject to meeting quality guidelines. At the same time, existing work suggests that most ACOs still do not offer hospital-based palliative care and even fewer provide community-based palliative care.[33, 34] These findings could reflect weak incentives to manage care at the end of life or perhaps the possibility that savings at this stage may be more difficult than many believe.[35, 36]
Additionally, Medicare is testing a new model of care—Medicare Choices—that would allow patients to continue receiving curative care once they enter hospice care—an option that has been unavailable until now.[37] This program is already available to children through the Medicaid program. If successful and scaled in Medicare, the approach may help expand palliative care to more seriously ill patients near the end of life who want to continue curative care.
Evidence From Systematic Reviews on Health Outcomes
The evidence base for palliative care is large and growing. However, the variation in palliative care programs and the populations receiving palliative care often make it difficult to generalize findings. One systematic review identified 43 randomized controlled trials (RCTs) studying the impact of palliative care on patient and/or caregiver outcomes.38 While the trials covered a range of settings for palliative care (home, ambulatory or hospital), most (N=30) included patients with cancer and/or heart failure (N=14). Despite variation in setting, the meta-analysis concluded that palliative care interventions were associated with improvements in patient quality of life and symptom burden. Effects were less clinically meaningful, although still present, when analysis was restricted to trials the study deemed at low risk of bias (N=5).
In support of NCP palliative care guidelines, Ahluwalia, et al., conducted an extensive survey of systematic reviews of palliative care research, including studies targeting patients with advanced, late- or end-stage, or metastatic illness or one of these groups in a subset analysis. Included studies had to report the impacts of a palliative care intervention on patients and/or family/caregiver outcomes and incorporate a comparator group (e.g., usual care). Based on a search of studies from 2013 on, the review found 139 high-quality systematic reviews alone, a signal of the vibrant and growing literature in this field. The review evaluated the palliative care literature in relation to eight research domains:
- Structure and processes of care
- Physical aspects of care
- Psychological and psychiatric aspects of care
- Social aspects of care
- Spiritual, religious and existential aspects of care
- Cultural aspects of care
- Care of the patient nearing the end of life
- Ethical and legal aspects of care
Numerous RCTs demonstrate the value of palliative care services in improving quality of life for patients and caregivers, care satisfaction, end-of life care communication and planning, and reducing unwanted treatments. One challenge in putting the evidence together, however, is that what constitutes palliative care—including but not limited to the design of the palliative care team, the services provided by the team and the care setting—can vary widely across studies. In addition, because of the difficulty of recruiting and following patients at the time of palliative care need, studies often are quite small.
Using the Grading of Recommendations, Assessment, Development and Evaluations framework,[39] Ahluwalia, et al., found high-quality evidence for only one domain of palliative care—the impact of home-based palliative care on the likelihood that patients die at home. That assessment was based on two systematic reviews covering multiple RCTs.[40, 41] Both reviews reported systematically higher rates of dying at home for patients in home-based palliative care relative to usual care, where usual care varied by context but in some cases included care at home but without specialized interdisciplinary teams. The most recent review in 2016 concluded that the likelihood of dying at home was about 33% higher for those receiving home-based palliative care, 95% CI [14%, 55%].
The review found moderate quality evidence for many other intended effects of palliative care, including the impact of:
- Interdisciplinary care teams on quality of life, advance care planning (ACP), death at home and patient/family life satisfaction
- Telehealth for adults and children in the home outpatient setting on psychological health
- Music/art therapy on pain management outcomes, anxiety and depression
- Comprehensive palliative care teams for adults on symptom burden
- Review or dignity therapy on spiritual wellbeing
- Grief and bereavement support on grief outcomes for children
- Ethics consultations in the intensive care unit (ICU) on clinical decision consensus
- Advance directive interventions on preference- concordant care
- Care planning discussions on preference-concordant care.[i]
In most of these cases, notably interdisciplinary care teams and quality of life, the existing evidence is consistently positive, and the moderate evidence rating is based on either some individual study limitations, variation in study design and/or a lack of pooled estimates across studies.
A more recent review,[42] focused specifically on inpatient palliative care consultation and the impact on post-discharge outcomes, including discharge to home or hospice; coordinated care; and hospital readmissions. Evaluating studies from 2000 to 2018 using a different assessment rubric, the Effective Public Health Practice Project tool, the review identified seven strong studies, four moderate and four weak studies. The review concluded that most studies found that patients with an inpatient palliative care consultation were more likely to be referred to hospice than those receiving usual care, had lower total costs of care and reported greater satisfaction with their care experience.[43]
Perhaps the most important lessons from the reviews are that, while clear support exists for some positive impacts of palliative care on patient and system outcomes, the devil is in the details. The variation in palliative care programs and the populations receiving palliative care often makes it difficult to generalize.
Economic Evaluation of Palliative Care
While the evidence base demonstrating that palliative care contributes to better health outcomes is growing, less of an evidence base is available that relates to economic outcomes. Moreover, much economic research related to palliative care centers on the end of life. While palliative care is suitable for all patients with serious illnesses, studying care at the end of life can increase our understanding of where palliative care is most needed and whether timely palliative care can decrease intensive interventions at the end of life.
Healthcare spending also is concentrated at the end of life, with the 1% of people who die annually in high-income countries accounting for about 10% of healthcare spending.[44] For example, Figure 2 shows that Medicare beneficiaries who died in 2014 had average costs approximately four times higher than those who did not die, with 4% of beneficiaries who died accounting for about 14% of expenditures.
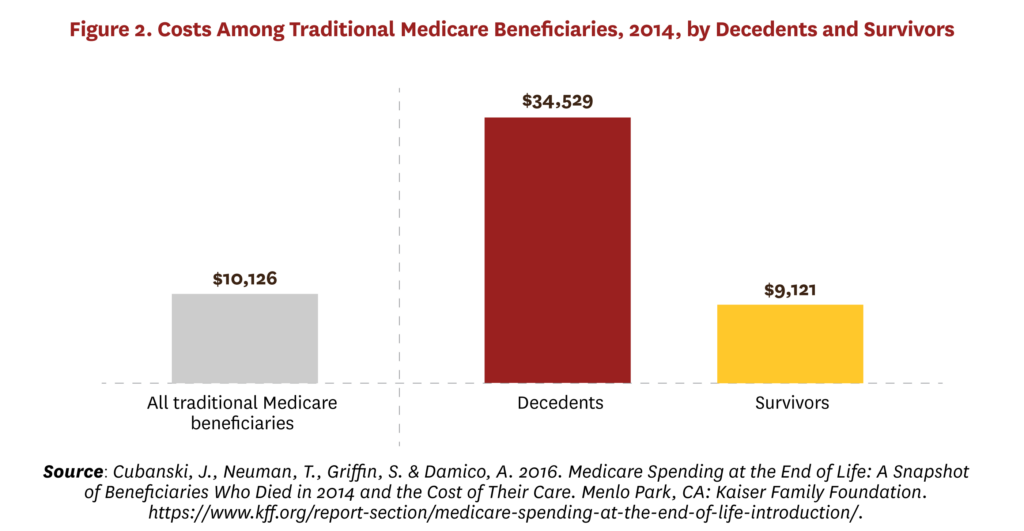
Economists have long been interested in the high costs associated with the last year of life, when many people face serious illnesses.[45, 46] To economists, high costs near end of life may appear wasteful or irrational. Economic theory states that one should only pay for things when the benefits are at least as great as the costs. Further, one should pay only when the ratio of benefits to costs is larger than for other options that could be pursued with available resources. Intuitively, it may seem that available benefits are low near end of life. At the individual level, high-cost treatments at the end of life are generally burdensome with an inherently limited timeframe to recoup benefits. At the societal level, spending large sums on people with limited life expectancy may be hard to justify when younger populations with a longer time to benefit are unable to access needed care.
However, microeconomic theory shows that, under certain assumptions, high end-of-life costs may be rational.[47, 48] Briefly, these assumptions rely on different ways that willingness to pay for healthcare may be magnified in the face of death, including negligible opportunity costs (“you can’t take it with you”), the value of hope (“what have I got to lose?”),[49] unmeasured third-party effects (e.g., improved experience for family members) and the increased “value” of life as death becomes nearer. For example, a recent discrete choice experiment in Switzerland evaluated willingness to pay for a novel cancer drug that required substantial out-of-pocket payment, and the assumptions received general empirical support.[50]
At the same time, a wide range of health services research suggests that this theory is not generalizable to most experiences with serious illness. At the population level, a majority of deaths occur due to diseases other than cancer. This means that, generally speaking: 1) deaths are not predictable long in advance;[51] 2) people are not dying “of” a single treatable disease but “with” multiple chronic conditions where the final cause of death is often not meaningfully identifiable or directly treatable;[52, 53] 3) the bulk of spending is not on high-risk, high-reward pharmaceutical gambles but rather burdensome transitions and unplanned hospital admissions;54, 55 and 4) where treatments are high cost and high intensity, add to patient burden (e.g., stenting, dialysis, ventilation), often started without eliciting patient preferences[56] and with poor physician understanding of patient preferences.[57]
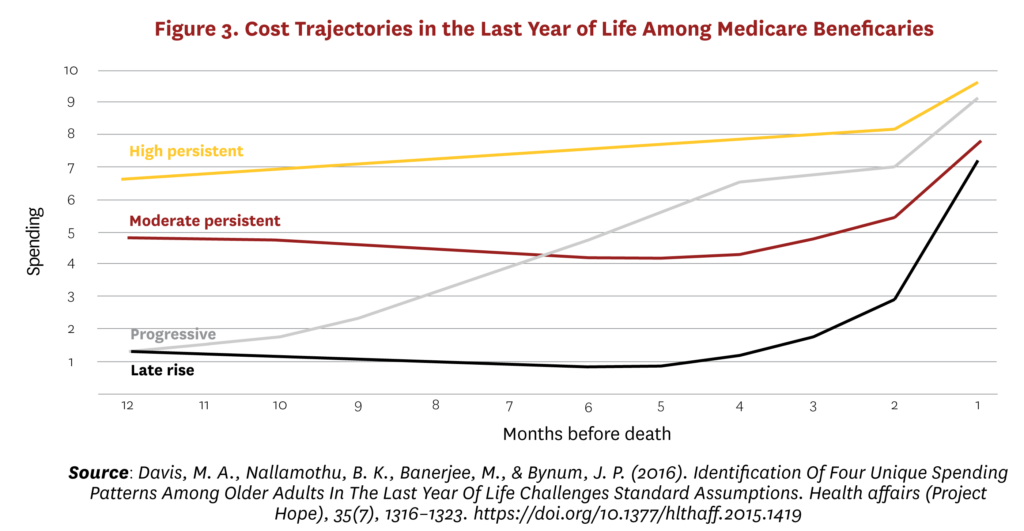
Therefore, high end-of-life costs do not so much reflect the preferences of rational and well-informed patients as the failure of healthcare systems to recognize and address patient preferences. Moreover, this is not an issue of the last days of life per se. Figure 3 shows four main trajectories of costs in the last year of life among Medicare beneficiaries.58 The highest cost individuals in the last year of life are not those for whom costs rise rapidly in response to a catastrophic diagnosis, but those who have persistently high costs through the last 12 months of life and in many cases the preceding years. They are not characterized by any specific diagnosis but by overall multiple disease burden. They represent the largest group in absolute numbers as well as in average costs among Medicare decedents, and a large majority would likely have benefited from palliative care.
Moreover, demographic aging means that the proportion of Medicare expenditures attributable to the end-of-life phase has been dropping consistently for 30 years, from 6% of decedents accounting for 30% of expenditures in the 1970s and 1980s59 to 4% of decedents accounting for 14% in 2014 (Figure 4).
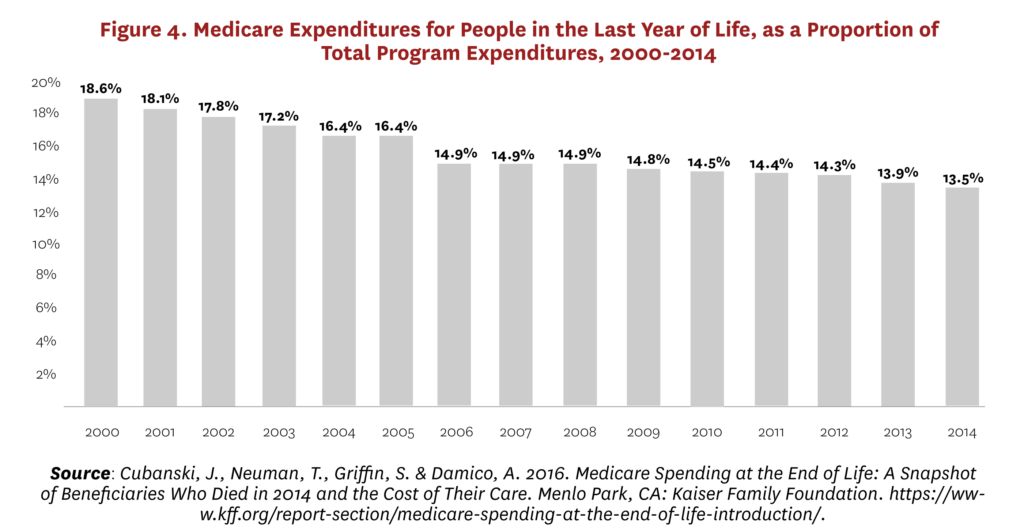
More people are joining Medicare each year than are dying, and the new joiners will live longer than their predecessors but have a higher burden of serious chronic disease. In 2011, the 16% of beneficiaries with six or more chronic conditions accounted for 47% of total program expenditures.[60]
Taken together, Figures 3 and 4 imply that the policy-relevant population for palliative care is the large and growing number of older people living and dying with multiple chronic conditions, complex needs, poor outcomes and high costs. This group has the highest mortality risk, and many will die in any given year. However, many more will not die in that year, and we cannot reliably predict deaths long in advance and, even if we could, end-of-life costs per se are less and less the policy problem. Instead, we must identify how to care not only for the growing share of people who will need medical care but also social and other supports to address the functional, psychological, practical and spiritual consequences of a serious illness.
Existing Economic Evaluation of Palliative Care
Economic evaluation is interested, for a given time period and from a given perspective, in the measurement of costs and outcomes for an intervention and its comparator. The timeframe should be long enough to capture all meaningful differences in costs and outcomes between treatment and comparison groups. Perspective relates to whose outcomes are measured, for example, costs to the payer or the provider or society as a whole. Quality of life is usually measured for the patient but also potentially for family members or attending staff. Any comparison can in principle be quantified in terms of effect on both costs and outcomes. Application of this method varies among countries according to local systems and political economy, but the intellectual fundamentals are consistent.[61, 62]
Scarcity means that there will never be sufficient money to meet all healthcare needs, and decisions must be made to fund some services and not others. Economic evaluation aims to inform these decisions by measuring both the resources required (what does this cost us?) and the health and survival benefits (what do we get for our money?). These benefits are most often captured in the quality-adjusted life year (QALY), which aims to combine effects on quality of life and survival into a single metric for comparison across different treatments. While most QALY applications are for specific drugs where relevant data are more readily available, there is no reason in principle why the approach cannot be applied to complex interventions63 and to changes in organization and delivery.[64]
Current evidence. Multiple literature reviews have addressed palliative care’s effects on cost and utilization. Some have focused on a specific setting, such as home care[65] or hospital care;[68, 69] others on specific populations such as late-stage cancer;[66, 67] and others on all interventions and populations.[70, 71] Four common themes emerge across these reviews.
- First, “full” economic evaluations of outcomes and costs are unusual. Studies mostly estimate effect on costs based on a non-inferiority assumption that outcomes are always at least as good for palliative care patients as comparison group patients.
- Second, studies do not prospectively follow participants for the full timeframe of interest, for example, from diagnosis or palliative care initiation to death. Instead, studies focus heavily on hospital care and the end-of-life phase.
- Third, perspective on costs tends to be narrow. Studies mostly assess routinely collected costs from the hospital or payer perspective.
- Fourth, palliative care appears to be cost saving. Taken in conjunction with the evidence that palliative care improves outcomes, this suggests the possibility for palliative care to be a dominant strategy (reducing costs and improving outcomes) in at least some circumstances.
Cost savings. Most reviews report cost savings in the 5%-20% range, although reviews differ in their quality threshold, and the estimated effects are smallest among the reviews with the highest threshold.[72, 73] These savings appear to accrue through a combination of reduced treatment intensity—for example, fewer tests and procedures, less invasive and high-intensity care near the end of life—and also better integrated care, such as expedited hospital discharge, connection of home care and hospice, and reduced readmissions. However, the evidence base is much smaller on informal care and out-of-pocket costs,[74] so it is mostly unknown how much observed savings reflect true improvements and how much they reflect cost shifting from formal care settings to the family and household.
Econometric analyses of palliative care. The reviews cited previously, and the included studies, were published predominantly in the medical literature. The most common research design is a cohort study: Investigators identify an exposure group that received palliative care and compare outcomes to a comparison group that did not receive it.[75] Matching on observed characteristics is often performed using propensity scores and unobserved confounding is unaddressed. Less consideration has been given to other economic and econometric approaches, and in particular those that are concerned with managing unobserved confounding. For example, program evaluation methods such as difference-in-differences and interrupted time series analysis are widely used to derive causal evidence in fields that rely heavily on routine observational data.[76]
We conducted a rapid review of the literature to identify use of econometrics in palliative and end-of-life populations. We searched EconLit and PubMed using keyword search terms relevant to these populations (e.g., palliative care, hospice care, terminal care) and methods (e.g., causal inference, difference-in-differences, instrumental variable). Only peer-reviewed articles written in English were included in the search results.
In EconLit, we searched using keywords palliative care, hospice care, terminal care and without any publication date restriction. Among 110 identified articles, 19 articles were selected as relevant to palliative care and economic evaluation after title and abstract screening. We took a more restrictive approach in PubMed given the enormous volume of palliative care articles it contained. Specifically, we searched with combined keywords: palliative care & causal inference, hospice care & causal inference, terminal care & causal inference, palliative care & difference-in-differences, hospice care & difference-in-differences, terminal care & difference-in-differences, palliative care & instrumental variable, palliative care & propensity score & cost, and palliative care & cost. For the keywords “palliative care & cost,” the search was restricted to articles published after January 1, 2018. More than a thousand (1,013) articles were identified with the abovementioned keywords; among them, 89 articles were selected as being relevant to the topics of interest after title and abstract screening. The list of relevant articles included studies of general and specific palliative care programs as well as a focus on general populations or populations with specific health conditions.
After de-duplication, we included a total of 100 studies—48 focused on “the economics” of palliative care for populations generally, 25 on specific health conditions (e.g., cancer) or populations (e.g., pediatric), six on very specific palliative care models (e.g., the Four Seasons/Duke CMMI Demonstration), 12 on palliative care consultations, and nine on hospice. The latter set of articles all used the term “hospice care” and “palliative care” interchangeably. A minority of the 100 articles were either 1) published in the economic literature or 2) grappled with the trade-offs central to traditional economic analysis. The remainder focused on issues broadly construed as economics, such as healthcare costs and utilization, or used econometric methods for purposes of causal inference in observational data.
A significant econometric literature exists on the relationship between time to death and costs.[77, 78, 79, 80] The research centers on the extent to which high costs in aged populations are a function of age and increasing need in old age versus the extent to which high costs are instead determined by proximity to death. The distinction is important since we only die once and costs increase sharply at the end of life.[81] Future cost projections omitting time to death as an explanatory variable will be biased upward82 and misidentify age as the key driver of healthcare costs among aged populations.[83]
While this is important for population-level cost projections, it has the same limitations as other methods using data on the ex post dead. We cannot use in prospective decision-making, e.g., designing policy or triaging patients at risk of high costs or poor value care based on their time to death, since this variable is ex ante unknowable.
Applications of econometric methods to ex ante analyses and decision-making are noticeably less common. Our search identified two studies from the same research team using difference-in-differences to evaluate hospice programs. One evaluation of hospice care in nursing homes identified a decrease in intensity of treatments near end of life but an overall increase in spending.[84] The other found a $266 daily reduction in costs from hospice among Veterans Affairs patients with advanced lung cancer.
This wide variation in estimates is consistent with non-causal evidence on heterogeneous treatment effects. A prospective cohort study in advanced cancer found that palliative care has substantial dose effects on costs; the earlier in the episode of care it is initiated, the larger the cost-saving effect.[86, 87] A subsequent meta-analysis found that cost effects vary according to primary diagnosis and number of comorbidities: Effects appear larger for those with cancer and those with higher numbers of comorbidities (see Figure 5).[88]
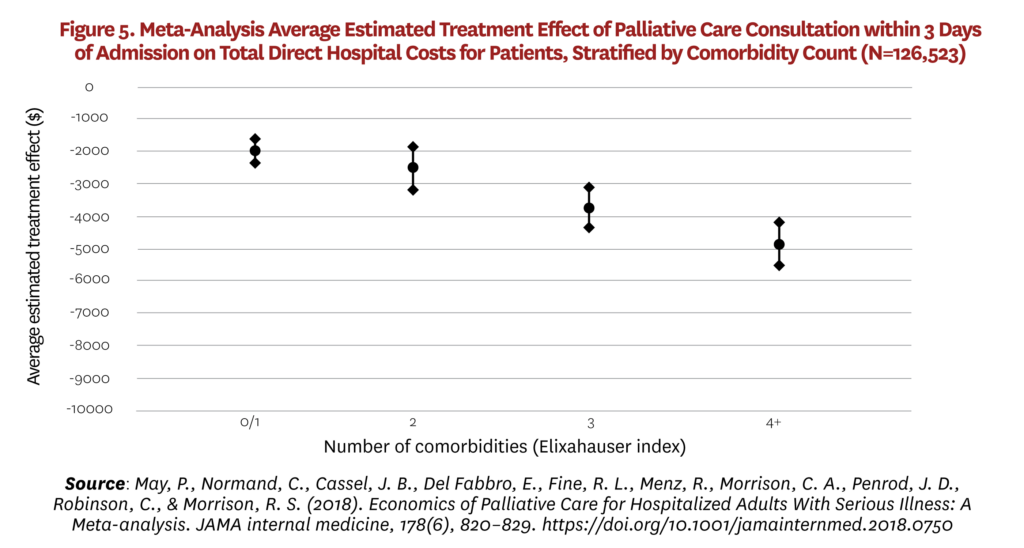
This variation is potentially important for a number of reasons. In populations where palliative care has a major impact on patterns of treatments and costs, earlier intervention is better. Palliative care does not simply reduce futile care in the last weeks of life but instead has benefits across the trajectory of disease in the management of complex patients. At the same time, effects are not so large in other groups and estimated savings from palliative care programs will be biased upward if investigators assume that savings will be consistent with the findings in some high-profile papers. While heterogeneous effects are not yet fully understood, it appears that savings are largest for advanced cancer patients, where prognosis and associated treatment options are relatively clear, and for those with more comorbidities, where the value of interdisciplinary decision-making may be greatest. As the nursing home evidence shows, it may not be so straightforward to eliminate futile care in older non-cancer patients with advanced frailty. In populations where cost effects are negligible, palliative care’s value is only then apparent through studies that measure outcomes alongside costs.
Why is the evidence base so small? Studying people living and dying with serious illness brings well-known challenges. Randomized trials face practical and ethical difficulties in recruitment and retention,[89] and the trials that are conducted are focused on the United States,[90] where studies are rarely sized appropriately to detect differences in costs data.[91] Primary data collection from people with high illness burden and, on average, heightened anxiety and distress is complex.[92] This leads to a heavy reliance on routinely collected observational data, which lack specificity for many research questions. Economic evaluation of interventions for people living and dying with serious illness brings additional challenges in the domains of time period, costs and outcomes.
Time period. One potential starting point is diagnosis of serious disease, an obvious inflection point for changing treatment choices. For example, the World Health Organization (WHO) and American Society for Clinical Oncology both recommend palliative care beginning at diagnosis.[93, 94] In observational data, treatment assignment is not under investigator control. Some subjects will start to receive palliative care at diagnosis as recommended and others will not, but these groups will differ on factors such as illness burden, prognosis and preferences. These factors are only partially captured in routine data, leading to pervasive selection bias risks.
An alternative approach is to start from death and work backward for a specified time. This narrows the sample to those definitively near the end of life and is an important methodology for research questions on end-of-life experience, where population representativeness is important. However, it has limited value for studies that seek to inform prospective decision-making, such as economics. First, we do not reliably know 12 months in advance who is entering the last year of life.[96] Second, an individual’s survival is potentially affected by their healthcare costs. Increased spending is often intended to extend life but, in some cases, may shorten it.96 Consequently treatment effect estimates derived from decedent cohort studies are subject to important biases.[97]
Costs. People with serious illness access healthcare in a wide range of settings for which they often have differential insurance coverage, and so costs are spread among payers, providers and patients in ways that are hard to observe and measure. This population also takes multiple medications, again with differential coverage. At a mundane practical level, the measurement of formal costs therefore requires complex data linkage and original collection to estimate and model out-of-pocket costs.
Perhaps most importantly, unpaid care costs are high among people with serious illness. Family and friends pay out of pocket on behalf of the person, provide care and transport, and forgo work. These caregivers are themselves susceptible to deteriorating health and workforce productivity both during the period of illness and in bereavement. Few of these factors are captured in any routine data.
Outcomes. All health research faces recurring challenges in outcome measurement, and these too may be exacerbated in palliative and end-of-life care. Outcomes are seldom recorded routinely, and tension remains in choosing between generic and context-specific measurement tools. Generic measures allow comparison of intervention effects across different populations and settings, e.g., through the QALY, but may not be sensitive to context and population. Specific measures should be more responsive to the population’s needs and outcomes but have limited comparability since by definition they are not widely used. Some have argued that this tension is particularly acute in death and dying, a unique life event in which preferences, priorities and the value of time may be altered.[98, 99] The opposing view in this “QALY problem” debate is that all health services research faces trade-offs in choosing tools and collecting outcome data, and seeking exemption from cost-effectiveness comparison across the health system represents special pleading.[100]
Finally, a fundamental challenge persists in defining the intervention under study. Palliative care is an inherently interdisciplinary and multifaceted concept. Detailed guidelines exist to define the structure and processes of care,[101] and these may inform study design; for example, a hospital consultation team at minimum should comprise a physician, a nurse and a social worker.[102] However, the exact composition of teams, and the ratio of different team members to patients seen, inevitably varies by hospital and system. A marked lack of evidence exists on the specific effect of individual team members or team composition on economic outcomes.
The scope for economics to improve the evidence base is clear. However, these efforts must proceed knowing weaknesses in the evidence base are not random or arbitrary. Current gaps reflect where and when data are collected: routinely, in hospitals and during the end-of-life phase. This is demonstrated by a few recent examples of difference-in-differences in the medical literature using decedent cohort studies,[103, 104] and other before-and-after designs evaluating single-site programs.[105, 106] On the one hand, these show that awareness of quasi-experimentation for causal inference is growing, but these studies must still tackle the practical challenges of sample definition, data collection and linkage, and outcome measurement.
Overview of the Literature on the Demand for Palliative Care
As palliative care continues to expand, especially in non-hospital settings such as physician offices, clinics, nursing homes and community settings, identifying future population-level demand remains critical for policy and planning purposes. The following summarizes how researchers have addressed questions on the current and future demand for palliative care.
International context. Relative to the U.S., international methods for quantifying the population-level demand for palliative care are well established and rely on the epidemiological approach of needs assessment, where need is defined by the population’s ability to benefit from healthcare.[108, 109] Typically, researchers have utilized mortality statistics,[110] in conjunction with methods that involve applying a fixed proportion to the total number of deaths in a population,[111] use of expert panels to identify the diseases and conditions most likely to benefit from palliative care,[112] and incorporation of data on hospital admissions or symptom prevalence.[113, 114, 115] Others have expanded palliative care needs assessment primarily by considering different or additional diseases or medical conditions and by developing a range of estimates to reflect levels of need.[115, 116, 117] Murtagh, et al., refined this method by consulting with an expert panel involving palliative care, primary care and public health practitioners.118 They analyzed a more detailed breakdown of the ICD-10 codes to address over- and under-counting of potential patients in need of palliative care and included a wider range of non-cancer conditions, such as dementia. Both methods are widely used in the international literature on the demand for palliative care. Alternatively, researchers in Spain considered patients with chronic progressive diseases and a limited life prognosis, which accounted for three-fourths of all deaths.[119, 120] Overall, the methodologies presented in the international literature progressed as palliative care became more widely accepted.
While these studies provided the groundwork for understanding and defining palliative care need, they do not project future demand, an exercise important to understand policy and planning considerations. Etkind, et al., modeled palliative care demand for England and Wales up to 2040 using two methods.[121] First, they assumed a constant proportion of deaths requiring palliative care (75%) and applied this estimate to the official national population and mortality projections, which account for long-term trends in fertility, mortality and migration. Second, they quantified palliative care needs according to age- and sex-specific deaths from a set of chronic diseases and applied this estimate to mortality projections in two separate calculations. Each method illustrated a growing need for palliative care in England and Wales.
In another advancement in the international literature on palliative care, the Lancet Commission on Global Access to Palliative Care and Pain Relief created a new construct of serious health-related suffering and estimate the global need for palliative care and pain relief.[122] The commission used mortality data for 20 conditions and adjusted for the prevalence of both physical and psychosocial symptoms to determine palliative care need, finding that more than 25 million people who died in 2015 experienced serious health-related suffering and may have benefited from palliative care. Using the Lancet Commission definition and WHO mortality projections, Sleeman, et al., projected the global burden of serious health-related suffering requiring palliative care through 2060.[123] The researchers projected the future demand for palliative care in five-year increments from 2020, with the proportion of people dying with serious health-related suffering calculated using the projected number of deaths as the denominator, concluding that the global burden of serious-health related suffering will nearly double by 2060.[124] For a complete list of medical conditions used to define palliative care need across these studies, see Appendix A.
United States context. In the U.S., demand for palliative care has been measured by examining healthcare utilization, costs and eligibility.[125, 126, 127, 128, 129, 130] For example, Hua, et al., measured palliative care need in the ICU by using screening criteria for palliative care consultation developed by an interdisciplinary group of experts for the ICU Project Advisory Board.[131] Development of the screening criteria involved literature review and search of the Center to Advance Palliative Care website for relevant tools.[132] The primary triggers identified for needing palliative care include: ICU admission after hospital stay of 10 or more days, 80 years or older with two or more life-threatening comorbidities, diagnosis of active state IV malignancy (metastatic disease), status after cardiac arrest and diagnosis of intracerebral hemorrhage requiring mechanical ventilation.[133] The authors found that about one in seven ICU admissions met these criteria.
Kelley and colleagues similarly focused on defining and operationalizing serious illness for the purpose of identifying palliative care need.134 They also utilized expert consensus and tested a range of definitions composed of diagnoses, utilization and markers of care needs. The authors also used additional claims-based elements to assess advanced stages of these diseases. To test utilization as a definition of need, the authors examined acute care, home health and skilled nursing facility use in the past six months, as well as durable medical equipment claims to measure severity of illness and functional impairment. Additional measures of need based on expert panel consensus included: functional dependence, nutritional decline, cognitive impairment, symptoms and caregiver strain. Despite this progress in defining the potential palliative care population, research priorities still include establishing a gold standard for defining palliative care need in a broad population and within specific settings (e.g., community versus hospital).
Modeling Palliative Care Supply/Demand in the U.S.
Estimates of current need and projections of future need have resulted from an assessment of the workforce tasked with providing palliative care services.[135, 136, 137, 138] For example, in its 2015 report on end of life care, the National Academy of Medicine cited one study estimating the national shortage of palliative care specialists to be between 6,000 and 18,000 physicians.[139, 140] To estimate the need for palliative care physicians, this study modeled hypothetical national demand using data on observed physician use (measured by patient days, admissions and hospice census size) at three hospice-based and one hospital-based palliative care institutions.[141] The study, however, does not identify population-level demand as it was not empirically determined and only considers demand at exemplar institutions.[142]
Lupu, et al., built on this work, applying a population-based approach to project the geographic distribution of the physician supply over time, taking into account annual changes in the labor force.[143] In this study, the authors used the population older than age 65 as a proxy for palliative care need to model the need for supply through 2040. Kamal, et al., also only consider the presence of one or more chronic conditions[144] or Medicare eligibility[145] in their measures for palliative care need to estimate future supply. Given that up to 50% of palliative care consultations involve people under the age of 65, the use of age or Medicare eligibility as a proxy for palliative care need is a key limitation. Notably, scarce research exists related to projecting palliative care workforce needs for other than physicians.
To our knowledge, no study has utilized a dynamic microsimulation model such as the Future Elderly Model (FEM) or Future Adult Model (FAM)ii to project future need for palliative care in the U.S. For two decades, the Health Policy Microsimulation team at the Schaeffer Center for Health Policy & Economics has been developing economic-demographic models to answer salient policy questions surrounding health and aging in the U.S. Findings have been featured by several government agencies, the White House and private organizations and have resulted in over 80 peer-reviewed manuscripts. While the FEM or FAM have not been explicitly used to forecast the future demand of palliative care, the models have been extensively used to project highly relevant future health and related economic outcomes for the elderly. In fact, one of the first papers to use the FEM forecasted growth in the U.S. nursing home population as a function of trends in marriage and disability.[146]
The Health Policy Microsimulation team has used the FEM and FAM to model complex health dynamics related to aging, such as the presence of comorbidities, that elevate mortality risk, as well as create the frailty and disability profile that can accompany old age and require palliative care.[147] The team has quantified the future burden of a multitude of chronic conditions that prominently define the need for palliative care in the literature. These conditions include cancer (breast, prostate, colorectal and lung), Alzheimer’s disease and related dementias, stroke, heart failure, chronic heart disease, renal failure for diabetes, chronic obstructive pulmonary disease and HIV/AIDS, among others. The FEM and FAM have also been used to project the future burden of functional limitations and pain, additional indicators for palliative care need.
Much of the palliative care literature that currently exists relies on administrative data for cause of death and simplifies future projections in mortality as linear functions of prior mortality trends or utilizes assumptions based on the current and projected workforce. The FEM or FAM may thus provide improved estimates of future palliative care demand as the models simulate future health trends and mortality based on those health trends rather than through historical time series.[148] Further, the FEM and FAM’s ability to model functional status, as well as a variety of economic outcomes, may allow for modeling novel and varied definitions of palliative care need. As the population ages and health status worsens, the need for palliative care will continue to grow and new methods, such as those incorporating the FEM, will provide policymakers with a greater understanding of that need and how to meet it.
Conclusion
Early assessments hint at the potential of increased use of palliative care to improve quality and reduce costs—or increase the value of U.S. healthcare spending. However, a critical need persists to close gaps in the literature by identifying key research questions related to palliative care and conducting economic research to build the evidence base for palliative care. The fragmented and complex nature of the U.S. healthcare system presents challenges to acquiring data to conduct rigorous economic research to advance palliative care. With the objective of identifying research gaps and novel projects to fill the gaps, the advisory panel will develop a prioritized research agenda to fill in the missing analysis that public and private payers need to increase access to palliative care across settings and populations.
Creating a palliative care research agenda can help lay the foundation for building a sound evidence base to guide public policy goals designed to increase the value of U.S. healthcare for patients, providers, payers and the public. The following outlines key initial questions to guide the advisory panel discussion:
- What should be the core priorities guiding economic research on palliative care?
- Where are the evidence gaps that present barriers to advancing best practices?
- What elements are needed to make value-based assessments of palliative care?
- What are the impacts on future trends in cost and quality of life outcomes with and without implementation of palliative care approaches?
- What economic evidence informed palliative care policy and practice in other countries?
- What models of palliative care might solve the multiple transitions in healthcare setting and fragmentation of care experience in the U.S.
- Have other countries successfully employed outcomes metrics that capture the cost-benefits of palliative care?
- How do we change incentives (for providers, payers, and patients and their families) and quality standards to optimize and scale palliative care?
- What types of clinical and reimbursement systems are appropriate for palliative care?
- What are the barriers, economic and otherwise, from the provider, payer and patient and family perspectives?
- What economic research can inform a new pathway for palliative care nationwide?
- What policy levers are available to support the scaling of palliative care nationwide?
- What data should be used for future research?
Support for this work was provided by the Gordon and Betty Moore Foundation, USC Schaeffer Center for Health Policy & Economics, and Cedars-Sinai. The views expressed herein are those of the authors, and do not represent the views of the funders. The Schaeffer Center is supported by a wide variety of public and private entities and donors.
This paper has undergone the Schaeffer Center white paper quality assurance process, led by Emmett Keeler, Schaeffer Center Senior Fellow and Quality Assurance Director. In addition to his review, the paper was reviewed by two scholars not affiliated with the Schaeffer Center.
The authors thank Narae Kim and Seema Passar for their contributions to this paper.
i Ahluwalia, et al,. (2018) found low to very low quality evidence for the impact of 1) telehealth on resource use, patient quality of life, physical symptoms, satisfaction with care, family/caregiver quality of life; 2) early/integrated care on most outcomes; 3) home-based palliative care on physical symptoms, quality of life, and family/caregiver burden and satisfaction; and 4) home-based palliative care on physical symptoms and resource use.
ii The most recent version of the FEM models trends in health, functional status, health spending, pharmaceutical innovation, labor supply and earnings for individuals over age 50 in the United States. The FAM is an extension of the FEM to ages 25 and over.
Appendix A
Table 1. Disease and Conditions Defining Need for Palliative Care in the International Literature (Adapted from Murtagh, Et., Al., 2014)
| Author | Diseases or conditions included in study |
| Higginson, 1997 | All cancer deaths
Non-cancer deaths from disease of circulatory system, respiratory sy tem, chronic liver and cirrhosis, nervous system and sense organs (including Parkinson’s disease, multiple sclerosis and meningitis), senile and pre-senile conditions, and endocrine, nutritional, metabolic and immunity disease |
| Rosenwax, et al., 2005 | All cancer deaths (C00-D48) – both malignant and benign neoplasms included
Non-cancer deaths: Heart failure, renal failure, alcoholic liver disease and chronic or unspecified hepatic failure, chronic obstructive pulmonary disease, neurodegenerative disease (motor neurone disease, Parkinson’s disease, Huntington’s disease), Alzheimer’s disease, HIV/AIDS ICD-10 codes: I500, I501, I509, I111, I130, I132, N180, N188, N189, N102, N112, N120, N131, N132, I132, K704, K711, K721, K729, J40, J410, J411, J418, J42, J430, J431, J432, J438, J439, J440, J441, J448, J449, G122, G20, G10, G300, G301, G308, G309, B20-B24 |
| Gomez-Batiste, et al., 2014 | All cause deaths
Patients living with advanced chronic disease and limited life prognosis Elderly with pluripathology and dependency |
| Murtagh, et al., 2014 | Elderly with dementia
Elderly living in nursing homes or homes for the elderly Disease-specific mortality All cancer deaths (C00-C97) – malignant neoplasms only included Non-cancer: Heart failure — chronic heart disease, hypertensive, ischaemic heart disease, cerebrovascular disease, renal failure (acute and chronic), renal ischaemia and infarction, renal malignancy and hypertensive renal disease, chronic liver diseases, chronic obstructive pulmonary disease, other chronic respiratory diseases, neurodegenerative disease – motor neurone disease, Parkinson’s disease, Huntington’s disease, multiple sclerosis, multi-system degenerative conditions, progressive supranuclear palsy, Alzheimer’s disease – early and late onset, unspecified Alzheimer’s disease (vascular dementia, unspecified dementia, all types of Alzheimer’s disease and senility), HIV/AIDS ICD-10 codes : I00-I52, I60-I69, N17, N18, N28, C64, I12, I13, K70-K77, J06-J18, J20-J22, J40-J47 and J96, G10, G20, G35, G122, G903, G231, F01, F03, G30, R54, B20-B24 |
| Etkind, et al., 2017 | All deaths from malignant neoplasms
Heart disease and heart failure, chronic lower respiratory disease, respiratory failure, reno-vascular disease, renal failure, liver disease, dementia, vascular dementia, Alzheimer’s disease, senility, Huntington’s disease, motor neurone disease, Parkinson’s disease, progressive supranuclear palsy, multiple sclerosis, multi-system atrophy, haemorrhagic, ischemic and unspecified stroke, HIV/AIDS |
| Sleeman, et al., (Lancet Commission), 2019 | Haemorrhagic fever, tuberculosis, HIV/AIDS, malignant neoplasms, leukemia, dementia, inflammatory central nervous system diseases, degenerative central nervous system diseases, cerebrovascular diseases, non-ischemic heart diseases, chronic ischemic heart diseases, lung diseases, liver diseases, renal failure, birth trauma, low birthweight and prematurity, congenital malformation, injury, musculoskeletal disorders, malnutrition |
References
- “Palliate.” Merriam-Webster.com Dictionary. Merriam-Webster; https://www.merriam-webster.com/dictionary/palliate. Accessed 12 Jul 2020.
- National Consensus Project for Quality Palliative Care. Clinical Practice Guidelines for Quality Palliative Care, 4th edition. Richmond, VA: National Coalition for Hospice and Palliative Care; 2018. https://www.nationalcoalitionhpc.org/ncp/. Accessed 12 Jul 2020.
- Ding, J., Johnson, C. E., & Cook, A. (2018). How We Should Assess the Delivery of End-Of-Life Care in General Practice? A Systematic Review. Journal of Palliative Medicine, 10.1089/jpm.2018.0194. Advance online publication. https://doi.org/10.1089/jpm.2018.0194.
- Ahluwalia, S. C., Chen, C., Raaen, L., Motala, A., Walling, A. M., Chamberlin, M., O’Hanlon, C., Larkin, J., Lorenz, K., Akinniranye, O., & Hempel, S. (2018). A Systematic Review in Support of the National Consensus Project Clinical Practice Guidelines for Quality Palliative Care, Fourth Edition. Journal of Pain and Symptom Management, 56(6), 831–870. https://doi.org/10.1016/j.jpainsymman.2018.09.008.
- Hanks, G. (2008). Palliative care: careless use of language undermines our identity. Palliative Medicine, 22(2), 109–110. https://doi.org/10.1177/0269216308089301.
- Hui, D., De La Cruz, M., Mori, M., et al. Concepts and definitions for “supportive care,” “best supportive care,” “palliative care,” and “hospice care” in the published literature, dictionaries, and textbooks. Support Care Cancer 21, 659–685 (2013). https://doi.org/10.1007/s00520-012-1564-y.
- von Gunten, C. F., & Lupu, D. (2004). Development of a medical subspecialty in palliative medicine: progress report. Journal of Palliative Medicine, 7(2), 209–219. https://doi.org/10.1089/109662104773709332.
- “Promoting Health for Older Adults.” Centers for Disease Control and Prevention. https://www.cdc.gov/chronicdisease/resources/publications/factsheets/promoting-health-for-older-adults.htm. Accessed Aug 2, 2020.
- Andrews, M. Demand Grows For Palliative Care. 2011, Kaiser Health News.
- Ahluwalia, et al., 2018.
- Growth of Palliative Care in U.S. Hospitals: 2018 Snapshot (2000-2016). 2018, Center to Advance Palliative Care.
- Institute of Medicine, 2015.
- Hawley P. (2017). Barriers to Access to Palliative Care. Palliative Care, 10, 1178224216688887. https://doi.org/10.1177/1178224216688887.
- Armour, S. “Coronavirus Crisis Drives Demand for Palliative Care.” April 2, 2020. https://www.wsj.com/articles/coronavirus-crisis-drives-demand-for-palliative-care-11585825201. Accessed Aug 3, 2020.
- Ballentine, J.M. (2020). “The Role of Palliative Care in a COVID-19 Pandemic.” Shiley Institute for Palliative Care. https://csupalliativecare.org/palliative-care-and-covid-19/#_ftn1.
- Bowman, B. A., Back, A. L., Esch, A. E., & Marshall, N. (2020). Crisis Symptom Management and Patient Communication Protocols Are Important Tools for All Clinicians Responding to COVID-19. Journal of Pain and Symptom Management, 60(2), e98–e100. https://doi.org/10.1016/j.jpainsymman.2020.03.028.
- Leiter, R.E. (2020). “Palliative care needs tweaking in the coronavirus era.” STAT. https://www.statnews.com/2020/05/13/redefining-palliative-care-coronavirus-era/.
- Andrasfay, T., & Goldman, N. (2021). Reductions in 2020 US life expectancy due to COVID-19 and the disproportionate impact on the Black and Latino populations. Proceedings of the National Academy of Sciences of the United States of America, 118(5), e2014746118. https://doi.org/10.1073/pnas.2014746118.
- Ding, et al., 2018.
- Kathpalia P., Smith A., & Lai, J.C. Underutilization of palliative care services in the liver transplant population. World J Transplant 2016; 6(3): 594-598 https://dx.doi.org/10.5500/wjt.v6.i3.594.
- Hayward, B. & Zappetti, D. (2019). Palliative Care For Patients With Advanced Chronic Obstructive Pulmonary Disease (COPD) Remains Underutilized, Clinical Pulmonary Medicine. 26(3) 102 https://doi.org/10.1097/CPM.0000000000000309.
- Hawley, P. (2017). Barriers to Access to Palliative Care. Palliative Care: Research and Treatment. https://doi.org/10.1177/1178224216688887.
- Agha,L., Frandsen,B. & Rebitzer,J.B. (2019). Fragmented division of labor and healthcare costs: Evidence from moves across regions. Journal of Public Economics, 169, 144-159, https://doi.org/10.1016/j.jpubeco.2018.11.001.
- Meier, et al., 2017.
- Taylor, D.H., Harker, M., Olson, A., Bull, J. “How Can We Increase The Use Of Palliative Care In Medicare? ” Health Affairs Blog. February 13, 2017. DOI: https://www.healthaffairs.org/do/10.1377/hblog20170213.058723/full.
- Cubanski, et al., 2016.
- Griffin, S., Cubanski, J., Neuman, T., Jankiewicz, A., Rousseau, D., & Kaiser Family Foundation (2016). Medicare and End-of-Life Care. JAMA, 316(17), 1754. https://doi.org/10.1001/jama.2016.15577.
- Neuman, P., & Jacobson, G. A. (2018). Medicare Advantage Checkup. The New England Journal of Medicine, 379(22), 2163–2172. https://doi.org/10.1056/NEJMhpr1804089.
- Huskamp, H. A., Buntin, M. B., Wang, V., & Newhouse, J. P. (2001). Providing care at the end of life: do Medicare rules impede good care? Health Affairs (Project Hope), 20(3), 204–211. https://doi.org/10.1377/hlthaff.20.3.204.
- Stevenson, D. G., & Huskamp, H. A. (2014). Integrating care at the end of life: should Medicare Advantage include hospice? JAMA, 311(15), 1493–1494. https://doi.org/10.1001/jama.2014.1018.
- Centers for Medicare & Medicaid Services. 2019. News Release. CMS Releases Final 2015 Medicare Advantage Encounter Data to Researchers. https://www.cms.gov/newsroom/press-releases/cms-releases-final-2015-medicare-advantage-encounter-data-researchers.
- Fields, T. & Silvers, A. 2020. Explaining the Newly-Released Medicare Advantage “Carve-In” Model. Center to Advance Palliative Care blog. https://www.capc.org/blog/explaining-hospice-benefit-medicare-advantage-carve-in-model/.
- Bleser, W. K., Saunders, R. S., Winfield, L., Japinga, M., Smith, N., Kaufman, B. G., Crook, H. L., Muhlestein, D. B., & McClellan, M. (2019). ACO Serious Illness Care: Survey and Case Studies Depict Current Challenges and Future Opportunities. Health Affairs (Project Hope), 38(6), 1011–1020. https://doi.org/10.1377/hlthaff.2019.00013.
- Driessen, J., & West, T. (2018). Recent Evidence on the Inclusion of Hospice and Palliative Care Physicians in Medicare Shared Savings Program Accountable Care Organization Networks. Journal of Palliative Medicine, 21(3), 373–375. https://doi.org/10.1089/jpm.2017.0325.
- Einav, et al., 2018.
- Meier, et al., 2017.
- “Medicare Care Choices Model.” Centers for Medicare & Medicaid Services. https://innovation.cms.gov/innovation-models/medicare-care-choices. Accessed Aug 3, 2020.
- Kavalieratos, D., Corbelli, J., Zhang, D., Dionne-Odom, J. N., Ernecoff, N. C., Hanmer, J., Hoydich, Z. P., Ikejiani, D. Z., Klein-Fedyshin, M., Zimmermann, C., Morton, S. C., Arnold, R. M., Heller, L., & Schenker, Y. (2016). Association Between Palliative Care and Patient and Caregiver Outcomes: A Systematic Review and Meta-analysis. JAMA, 316(20), 2104–2114. https://doi.org/10.1001/jama.2016.16840.
- Guyatt, G. H., Oxman, A. D., Kunz, R., Vist, G. E., Falck-Ytter, Y., Schünemann, H. J., & GRADE Working Group (2008). What is “quality of evidence” and why is it important to clinicians? BMJ (Clinical research ed.), 336(7651), 995–998. https://doi.org/10.1136/bmj.39490.551019.BE.
- Shepperd, S., Gonçalves-Bradley, D. C., Straus, S. E., & Wee, B. (2016). Hospital at home: home-based end-of-life care. The Cochrane Database of Systematic Reviews, 2(2), CD009231. https://doi.org/10.1002/14651858.CD009231.pub2.
- Gomes, B., Calanzani, N., Curiale, V., McCrone, P., & Higginson, I. J. (2013). Effectiveness and cost-effectiveness of home palliative care services for adults with advanced illness and their caregivers. The Cochrane Database of Systematic Reviews, (6), CD007760. https://doi.org/10.1002/14651858.CD007760.pub2.
- Scott, M., Shaver, N., Lapenskie, J., Isenberg, S. R., Saunders, S., Hsu, A. T., & Tanuseputro, P. (2020). Does inpatient palliative care consultation impact outcomes following hospital discharge? A narrative systematic review. Palliative Medicine, 34(1), 5–15. https://doi.org/10.1177/0269216319870649.
- Gade, G., Venohr, I., Conner, D., McGrady, K., Beane, J., Richardson, R. H., Williams, M. P., Liberson, M., Blum, M., & Della Penna, R. (2008). Impact of an inpatient palliative care team: a randomized control trial. Journal of Palliative Medicine, 11(2), 180–190. https://doi.org/10.1089/jpm.2007.0055.
- French, E. B., McCauley, J., Aragon, M., Bakx, P., Chalkley, M., Chen, S. H., Christensen, B. J., Chuang, H., Côté-Sergent, A., De Nardi, M., Fan, E., Échevin, D., Geoffard, P. Y., Gastaldi-Ménager, C., Gørtz, M., Ibuka, Y., Jones, J. B., Kallestrup-Lamb, M., Karlsson, M., Klein, T. J., … Kelly, E. (2017). End-Of-Life Medical Spending In Last Twelve Months Of Life Is Lower Than Previously Reported. Health Affairs (Project Hope), 36(7), 1211–1217. https://doi.org/10.1377/hlthaff.2017.0174.
- Scitovsky, A. A. (2005). “The high cost of dying”: what do the data show? 1984. The Milbank Quarterly, 83(4), 825–841. https://doi.org/10.1111/j.1468-0009.2005.00402.x.
- Kane, R. L., Wales, J., Bernstein, L., Leibowitz, A., & Kaplan, S. (1984). A randomised controlled trial of hospice care. Lancet (London, England), 1(8382), 890–894. https://doi.org/10.1016/s0140-6736(84)91349-7.
- Becker, G., Murphy, K., & Philipson, T. (2007), The Value of Life Near its End and Terminal Care, No 13333, NBER Working Papers, National Bureau of Economic Research Inc. https://EconPapers.repec.org/RePEc:nbr:nberwo:13333.
- Philipson, T., Becker, G., Goldman, D., & Murphy, K. (2010), Terminal Care and The Value of Life Near Its End, No 15649, NBER Working Papers, National Bureau of Economic Research Inc. https://EconPapers.repec.org/RePEc:nbr:nberwo:15649.
- Lakdawalla, D. N., Romley, J. A., Sanchez, Y., Maclean, J. R., Penrod, J. R., & Philipson, T. (2012). How cancer patients value hope and the implications for cost-effectiveness assessments of high-cost cancer therapies. Health Affairs (Project Hope), 31(4), 676–682. https://doi.org/10.1377/hlthaff.2011.1300.
- Fischer, B., Telser, H., & Zweifel, P. (2018). End-of-life healthcare expenditure: Testing economic explanations using a discrete choice experiment. Journal of Health Economics, 60, 30–38. https://doi.org/10.1016/j.jhealeco.2018.06.001.
- Einav, L., Finkelstein, A., Mullainathan, S., & Obermeyer, Z. (2018). Predictive modeling of U.S. health care spending in late life. Science (New York, N.Y.), 360(6396), 1462–1465. https://doi.org/10.1126/science.aar5045.
- Martín-Lesende, I., Recalde, E., Viviane-Wunderling, P., Pinar, T., Borghesi, F., Aguirre, T., Recio, M., Martínez, M. E., & Asua, J. (2016). Mortality in a cohort of complex patients with chronic illnesses and multimorbidity: a descriptive longitudinal study. BMC Palliative Care, 15, 42. https://doi.org/10.1186/s12904-016-0111-x.
- Danilova, I.A. Problems of the quality of cause-specific mortality statistics at old ages. Adv Gerontol 6, 1–5 (2016). https://doi.org/10.1134/S2079057016010021.
- Institute of Medicine. 2015. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: The National Academies Press. https://doi.org/10.17226/18748.
- Cubanski, J., Neuman, T., Griffin, S. & Damico, A. 2016. Medicare Spending at the End of Life: A Snapshot of Beneficiaries Who Died in 2014 and the Cost of Their Care. Menlo Park, CA: Kaiser Family Foundation. https://www.kff.org/report-section/medicare-spending-at-the-end-of-life-introduction/.
- Kelley, A. S., Wenger, N. S., & Sarkisian, C. A. (2010). Opiniones: end-of-life care preferences and planning of older Latinos. Journal of the American Geriatrics Society, 58(6), 1109–1116. https://doi.org/10.1111/j.1532-5415.2010.02853.x.
- Downey, L., Au, D. H., Curtis, J. R., & Engelberg, R. A. (2013). Life-sustaining treatment preferences: matches and mismatches between patients’ preferences and clinicians’ perceptions. Journal of Pain and Symptom Management, 46(1), 9–19. https://doi.org/10.1016/j.jpainsymman.2012.07.002.
- Davis, M. A., Nallamothu, B. K., Banerjee, M., & Bynum, J. P. (2016). Identification Of Four Unique Spending Patterns Among Older Adults In The Last Year Of Life Challenges Standard Assumptions. Health Affairs (Project Hope), 35(7), 1316–1323. https://doi.org/10.1377/hlthaff.2015.1419.
- Lubitz, J. D., & Riley, G. F. (1993). Trends in Medicare payments in the last year of life. The New England Journal of Medicine, 328(15), 1092–1096. https://doi.org/10.1056/NEJM199304153281506.
- Lochner, K. A., Goodman, R. A., Posner, S., & Parekh, A. (2013). Multiple chronic conditions among Medicare beneficiaries: state-level variations in prevalence, utilization, and cost, 2011. Medicare & Medicaid Research Review, 3(3), mmrr.003.03.b02. https://doi.org/10.5600/mmrr.003.03.b02.
- Neumann, P.J., Sanders, G.D., Russell, L.B., Seigel, J.E. & Ganiats, T.G. 2017. Cost effectiveness in health and medicine, Oxford; New York, Oxford University Press. https://global.oup.com/academic/product/cost-effectiveness-in-health-and-medicine-9780190492939?cc=us&lang=en&.
- Drummond, M.F., Sculpher, M.J., Claxton, K., Stoddart, G.L. & Torrance, G.W. 2015. Methods for the Economic Evaluation of Health Care Programmes, Oxford: Oxford University Press. https://global.oup.com/academic/product/methods-for-the-economic-evaluation-of-health-care-programmes-9780199665884?cc=us&lang=en&.
- Byford, S., & Sefton, T. (2003). Economic Evaluation of Complex Health and Social Care Interventions. National Institute Economic Review, 186, 98-108. https://doi.org/10.1177/002795010300100114.
- Meacock R. (2019). Methods for the economic evaluation of changes to the organisation and delivery of health services: principal challenges and recommendations. Health Economics, Policy, and Law, 14(1), 119–134. https://doi.org/10.1017/S1744133118000063.
- Gomes, et al., 2013.
- Khandelwal, N., Kross, E. K., Engelberg, R. A., Coe, N. B., Long, A. C., & Curtis, J. R. (2015). Estimating the effect of palliative care interventions and advance care planning on ICU utilization: a systematic review. Critical Care Medicine, 43(5), 1102–1111. https://doi.org/10.1097/CCM.0000000000000852.
- May, P., Garrido, M. M., Cassel, J. B., Kelley, A. S., Meier, D. E., Normand, C., Smith, T. J., Stefanis, L., & Morrison, R. S. (2015). Prospective Cohort Study of Hospital Palliative Care Teams for Inpatients With Advanced Cancer: Earlier Consultation Is Associated With Larger Cost-Saving Effect. Journal of Clinical Oncology: official journal of the American Society of Clinical Oncology, 33(25), 2745–2752. https://doi.org/10.1200/JCO.2014.60.2334.
- Langton, J. M., Blanch, B., Drew, A. K., Haas, M., Ingham, J. M., & Pearson, S. A. (2014). Retrospective studies of end-of-life resource utilization and costs in cancer care using health administrative data: a systematic review. Palliative Medicine, 28(10), 1167–1196. https://doi.org/10.1177/0269216314533813.
- Higginson, I. J., & Evans, C. J. (2010). What is the evidence that palliative care teams improve outcomes for cancer patients and their families? Cancer Journal (Sudbury, Mass.), 16(5), 423–435. https://doi.org/10.1097/PPO.0b013e3181f684e5.
- Smith, S., Brick, A., O’Hara, S., & Normand, C. (2014). Evidence on the cost and cost-effectiveness of palliative care: a literature review. Palliative Medicine, 28(2), 130–150. https://doi.org/10.1177/0269216313493466.
- Kavalieratos, et al., 2016.
- Gomes, et al., 2013.
- Kavalieratos, et al., 2016.
- Gardiner, C., Brereton, L., Frey, R., Wilkinson-Meyers, L., & Gott, M. (2014). Exploring the financial impact of caring for family members receiving palliative and end-of-life care: a systematic review of the literature. Palliative Medicine, 28(5), 375–390. https://doi.org/10.1177/0269216313510588.
- Smith, S. et al. 2014.
- Gertler, Paul J.; Martinez, Sebastian; Premand, Patrick; Rawlings, Laura B.; Vermeersch, Christel M. J.. 2016. Impact Evaluation in Practice, Second Edition. Washington, DC: Inter-American Development Bank and World Bank. © World Bank. https://openknowledge.worldbank.org/handle/10986/25030 License: CC BY 3.0 IGO.
- Zweifel, P., Felder, S., & Meiers, M. (1999). Ageing of population and health care expenditure: a red herring? Health Economics, 8(6), 485–496. https://doi.org/10.1002/hec.826.
- Werblow, A., Felder, S., & Zweifel, P. (2007). Population ageing and health care expenditure: a school of ‘red herrings’? Health Economics, 16(10), 1109–1126. https://doi.org/10.1002/hec.1213.
- Howdon, D., & Rice, N. (2018). Health care expenditures, age, proximity to death and morbidity: Implications for an ageing population. Journal of Health Economics, 57, 60–74. https://doi.org/10.1016/j.jhealeco.2017.11.001.
- Mcgrail, K., Green, B., Barer, M. L., Evans, R. G., Hertzman, C., & Normand, C. (2000). Age, costs of acute and long-term care and proximity to death: evidence for 1987-88 and 1994-95 in British Columbia. Age and Ageing, 29(3), 249–253. https://doi.org/10.1093/ageing/29.3.249.
- Stearns, S. C., & Norton, E. C. (2004). Time to include time to death? The future of health care expenditure predictions. Health Economics, 13(4), 315–327. https://doi.org/10.1002/hec.831.
- Werblow, et al., 2007.
- Gozalo, P., Plotzke, M., Mor, V., Miller, S. C., & Teno, J. M. (2015). Changes in Medicare costs with the growth of hospice care in nursing homes. The New England Journal of Medicine, 372(19), 1823–1831. https://doi.org/10.1056/NEJMsa1408705.
- Mor, V., Wagner, T. H., Levy, C., Ersek, M., Miller, S. C., Gidwani-Marszowski, R., Joyce, N., Faricy-Anderson, K., Corneau, E. A., Lorenz, K., Kinosian, B., & Shreve, S. (2019). Association of Expanded VA Hospice Care With Aggressive Care and Cost for Veterans With Advanced Lung Cancer. JAMA Oncology, 5(6), 810–816. https://doi.org/10.1001/jamaoncol.2019.0081.
- May, et al., 2015.
- Scibetta, C., Kerr, K., Mcguire, J., & Rabow, M.W. (2016). The Costs of Waiting: Implications of the Timing of Palliative Care Consultation among a Cohort of Decedents at a Comprehensive Cancer Center. Journal of Palliative Medicine, 19(1), 69–75. https://doi.org/10.1089/jpm.2015.0119.
- May, P., Normand, C., Cassel, J. B., Del Fabbro, E., Fine, R. L., Menz, R., Morrison, C. A., Penrod, J. D., Robinson, C., & Morrison, R. S. (2018). Economics of Palliative Care for Hospitalized Adults With Serious Illness: A Meta-analysis. JAMA Internal Medicine, 178(6), 820–829. https://doi.org/10.1001/jamainternmed.2018.0750.
- Ewing, G., Rogers, M., Barclay, S., McCabe, J., Martin, A., & Todd, C. (2004). Recruiting patients into a primary care based study of palliative care: why is it so difficult? Palliative Medicine, 18(5), 452–459. https://doi.org/10.1191/0269216304pm905oa.
- Kavalieratos, et al., 2016.
- Briggs, A. (2000). Economic evaluation and clinical trials: size matters. BMJ (Clinical research ed.), 321(7273), 1362–1363. https://doi.org/10.1136/bmj.321.7273.1362.
- Higginson, I. J., Evans, C. J., Grande, G., Preston, N., Morgan, M., McCrone, P., Lewis, P., Fayers, P., Harding, R., Hotopf, M., Murray, S. A., Benalia, H., Gysels, M., Farquhar, M., Todd, C., & MORECare (2013). Evaluating complex interventions in end of life care: the MORECare statement on good practice generated by a synthesis of transparent expert consultations and systematic reviews. BMC Medicine, 11, 111. https://doi.org/10.1186/1741-7015-11-111.
- World Health Organization. 2020. Definition of Palliative Care [Online]. https://www.who.int/cancer/palliative/definition/en/. Accessed Jul 13, 2020.
- Ferrell, B. R., Temel, J. S., Temin, S., Alesi, E. R., Balboni, T. A., Basch, E. M., Firn, J. I., Paice, J. A., Peppercorn, J. M., Phillips, T., Stovall, E. L., Zimmermann, C., & Smith, T. J. (2017). Integration of Palliative Care Into Standard Oncology Care: American Society of Clinical Oncology Clinical Practice Guideline Update. Journal of Clinical Oncology: official journal of the American Society of Clinical Oncology, 35(1), 96–112. https://doi.org/10.1200/JCO.2016.70.1474.
- Einav, et al., 2018.
- Temel, J. S., Greer, J. A., Muzikansky, A., Gallagher, E. R., Admane, S., Jackson, V. A., Dahlin, C. M., Blinderman, C. D., Jacobsen, J., Pirl, W. F., Billings, J. A., & Lynch, T. J. (2010). Early palliative care for patients with metastatic non-small-cell lung cancer. The New England Journal of Medicine, 363(8), 733–742. https://doi.org/10.1056/NEJMoa1000678.
- Bach, P. B., Schrag, D., & Begg, C. B. (2004). Resurrecting treatment histories of dead patients: a study design that should be laid to rest. JAMA, 292(22), 2765–2770. https://doi.org/10.1001/jama.292.22.2765.
- Normand C. (2009). Measuring outcomes in palliative care: limitations of QALYs and the road to PalYs. Journal of Pain and Symptom Management, 38(1), 27–31. https://doi.org/10.1016/j.jpainsymman.2009.04.005.
- Philipson, et al., 2010.
- Round, J. (2012). Is a QALY still a QALY at the end of life? Journal of Health Economics, 31(3), 521–527. https://doi.org/10.1016/j.jhealeco.2012.01.006.
- Ferrell, B. R., Twaddle, M. L., Melnick, A., & Meier, D. E. (2018). National Consensus Project Clinical Practice Guidelines for Quality Palliative Care Guidelines, 4th Edition. Journal of Palliative Medicine, 21(12), 1684–1689. https://doi.org/10.1089/jpm.2018.0431.
- May, et al, 2015.
- Chen, B., Kuo, C. C., Huang, N., & Fan, V. Y. (2018). Reducing costs at the end of life through provider incentives for hospice care: A retrospective cohort study. Palliative Medicine, 32(8), 1389–1400. https://doi.org/10.1177/0269216318774899.
- Hua, M., Lu, Y., Ma, X., Morrison, R. S., Li, G., & Wunsch, H. (2020). Association Between the Implementation of Hospital-Based Palliative Care and Use of Intensive Care During Terminal Hospitalizations. JAMA network open, 3(1), e1918675. https://doi.org/10.1001/jamanetworkopen.2019.18675.
- Chang, S., May, P., Goldstein, N. E., Wisnivesky, J., Ricks, D., Fuld, D., Aldridge, M., Rosenzweig, K., Morrison, R. S., & Dharmarajan, K. V. (2018). A Palliative Radiation Oncology Consult Service Reduces Total Costs During Hospitalization. Journal of Pain and Symptom Mmanagement, 55(6), 1452–1458. https://doi.org/10.1016/j.jpainsymman.2018.03.005.
- Tiernan, E., Ryan, J., Casey, M., Hale, A., O’Reilly, V., Devenish, M., Whyte, B., Hollingsworth, S., Price, O., Callanan, I., Walsh, D., Normand, C., & May, P. (2019). A quasi-experimental evaluation of an intervention to increase palliative medicine referral in the emergency department. Journal of Health Services Research & Policy, 24(3), 155–163. https://doi.org/10.1177/1355819619839087.
- Institute of Medicine, 2015.
- Bradshaw, J., Taxonomy of Social Need. 1972.
- Stevens, A., et al., Health care needs assessment: the epidemiologically based needs assessment reviews. Vol. 1. 2004: Radcliffe Publishing.
- Sleeman, K. E., de Brito, M., Etkind, S., Nkhoma, K., Guo, P., Higginson, I. J., Gomes, B., & Harding, R. (2019). The escalating global burden of serious health-related suffering: projections to 2060 by world regions, age groups, and health conditions. The Lancet. Global health, 7(7), e883–e892. https://doi.org/10.1016/S2214-109X(19)30172-X.
- Gómez-Batiste, X., Martínez-Muñoz, M., Blay, C., Amblàs, J., Vila, L., Costa, X., Espaulella, J., Espinosa, J., Constante, C., & Mitchell, G. K. (2014). Prevalence and characteristics of patients with advanced chronic conditions in need of palliative care in the general population: a cross-sectional study. Palliative Medicine, 28(4), 302–311. https://doi.org/10.1177/0269216313518266.
- Murtagh, F. E., Bausewein, C., Verne, J., Groeneveld, E. I., Kaloki, Y. E., & Higginson, I. J. (2014). How many people need palliative care? A study developing and comparing methods for population-based estimates. Palliative Medicine, 28(1), 49–58. https://doi.org/10.1177/0269216313489367.
- Ibid.
- Higginson, I., Palliative and Terminal Care. 1997: Radcliffe Medical.
- Gómez-Batiste, et al., 2014.
- Murtagh, et al., 2014.
- Rosenwax, L.K., McNamara, B., Blackmore, A.M., & Holman, C.D. (2005). Estimating the size of a potential palliative care population. Palliative Medicine, 19(7), 556–562. https://doi.org/10.1191/0269216305pm1067oa.
- Murtagh, et al., 2014.
- Gómez-Batiste, et al., 2014.
- Gómez-Batiste, X., Blay, C., Martínez-Muñoz, M., Lasmarías, C., Vila, L., Espinosa, J., Costa, X., Sánchez-Ferrin, P., Bullich, I., Constante, C., & Kelley, E. 2016. The Catalonia WHO Demonstration Project of Palliative Care: Results at 25 Years (1990-2015). Journal of Pain and Symptom Management, 52(1), 92–99. https://doi.org/10.1016/j.jpainsymman.2015.11.029
- Etkind, S. N., Bone, A. E., Gomes, B., Lovell, N., Evans, C. J., Higginson, I. J., & Murtagh, F. 2017. How many people will need palliative care in 2040? Past trends, future projections and implications for services. BMC Medicine, 15(1), 102. https://doi.org/10.1186/s12916-017-0860-2.
- Knaul, F. M., Farmer, P. E., Krakauer, E. L., De Lima, L., Bhadelia, A., Jiang Kwete, X., Arreola-Ornelas, H., Gómez-Dantés, O., Rodriguez, N. M., Alleyne, G., Connor, S. R., Hunter, D. J., Lohman, D., Radbruch, L., Del Rocío Sáenz Madrigal, M., Atun, R., Foley, K. M., Frenk, J., Jamison, D. T., Rajagopal, M. R., … Lancet Commission on Palliative Care and Pain Relief Study Group. 2018. Alleviating the access abyss in palliative care and pain relief-an imperative of universal health coverage: the Lancet Commission report. Lancet (London, England), 391(10128), 1391–1454. https://doi.org/10.1016/S0140-6736(17)32513-8.
- Sleeman, et al., 2019.
- Ibid.
- Lupu, D., & American Academy of Hospice and Palliative Medicine Workforce Task Force. 2010. Estimate of current hospice and palliative medicine physician workforce shortage. Journal of Pain and Symptom Management, 40(6), 899–911. https://doi.org/10.1016/j.jpainsymman.2010.07.004.
- Lupu, D., Quigley, L., Mehfoud, N., & Salsberg, E. S. 2018. The Growing Demand for Hospice and Palliative Medicine Physicians: Will the Supply Keep Up? Journal of Pain and Symptom Management, 55(4), 1216–1223. https://doi.org/10.1016/j.jpainsymman.2018.01.011.
- Kamal, A. H., Bull, J. H., Swetz, K. M., Wolf, S. P., Shanafelt, T. D., & Myers, E. R. 2017. Future of the Palliative Care Workforce: Preview to an Impending Crisis. The American Journal of Medicine, 130(2), 113–114. https://doi.org/10.1016/j.amjmed.2016.08.046.
- Kamal, A. H., Wolf, S. P., Troy, J., Leff, V., Dahlin, C., Rotella, J. D., Handzo, G., Rodgers, P. E., & Myers, E. R. 2019. Policy Changes Key To Promoting Sustainability And Growth Of The Specialty Palliative Care Workforce. Health Affairs (Project Hope), 38(6), 910–918. https://doi.org/10.1377/hlthaff.2019.00018.
- Kelley, A. S., & Bollens-Lund, E. 2018. Identifying the Population with Serious Illness: The “Denominator” Challenge. Journal of Palliative Medicine, 21(S2), S7–S16. https://doi.org/10.1089/jpm.2017.0548.
- Hua, M. S., Li, G., Blinderman, C. D., & Wunsch, H. (2014). Estimates of the need for palliative care consultation across united states intensive care units using a trigger-based model. American Journal of Respiratory and Critical Care Medicine, 189(4), 428–436. https://doi.org/10.1164/rccm.201307-1229OC.
- Ibid.
- Nelson, J. E., Curtis, J. R., Mulkerin, C., Campbell, M., Lustbader, D. R., Mosenthal, A. C., Puntillo, K., Ray, D. E., Bassett, R., Boss, R. D., Brasel, K. J., Frontera, J. A., Hays, R. M., Weissman, D. E., & Improving Palliative Care in the ICU (IPAL-ICU) Project Advisory Board. 2013. Choosing and using screening criteria for palliative care consultation in the ICU: a report from the Improving Palliative Care in the ICU (IPAL-ICU) Advisory Board. Critical Care Medicine, 41(10), 2318–2327. https://doi.org/10.1097/CCM.0b013e31828cf12c.
- Hua, et al., 2014.
- Kelley & Bollens-Lund, 2018.
- Lupu, D., & American Academy of Hospice and Palliative Medicine Workforce Task Force (2010). Estimate of current hospice and palliative medicine physician workforce shortage. Journal of Pain and Symptom Management, 40(6), 899–911. https://doi.org/10.1016/j.jpainsymman.2010.07.004.
- Lupu, D., Quigley, L., Mehfoud, N., & Salsberg, E. S. (2018). The Growing Demand for Hospice and Palliative Medicine Physicians: Will the Supply Keep Up? Journal of Pain and Symptom Management, 55(4), 1216–1223. https://doi.org/10.1016/j.jpainsymman.2018.01.011.
- Kamal, et al., 2017.
- Kamal, et al., 2019.
- Institute of Medicine, 2015.
- Lupu, 2010.
- Ibid.
- Institute of Medicine, 2015.
- Lupu, et.al, 2018.
- Kamal, et al., 2017.
- Kamal, et al., 2019.
- Lakdawalla, D., Goldman, D. P., Bhattacharya, J., Hurd, M. D., Joyce, G. F., & Panis, C. W. (2003). Forecasting the nursing home population. Medical Care, 41(1), 8–20. https://doi.org/10.1097/00005650-200301000-00003.
- Gaudette, É., Tysinger, B., Cassil, A., & Goldman, D. P. (2015). Health and Health Care of Medicare Beneficiaries in 2030, Forum for Health Economics & Policy, 18(2), 75-96. doi: https://doi.org/10.1515/fhep-2015-0037.
- Goldman, D.P., et al., 2015. The future elderly model: Technical documentation. University of South California. https://roybalhealthpolicy.usc.edu/files/2015/05/FEM_techdoc.pdf.
You must be logged in to post a comment.