In a hearing on how to balance incentives for medical innovation with patient access, USC Schaeffer Center Director of Research Darius Lakdawalla shared findings demonstrating that “expanding prescription drug coverage is worth the cost because it simultaneously rewards innovators and makes innovation more accessible.”
In testimony before the U.S. House Ways and Means Subcommittee on Health on May 10, Lakdawalla suggested targeted reforms to the Inflation Reduction Act (IRA) of 2022 as well as Centers for Medicare and Medicaid Services (CMS) programs that would improve the system for patients and innovators. Lakdawalla is noted for his studies on how to cost-effectively stimulate biomedical innovation so that as many patients as possible can benefit from its breakthroughs.
He was part of a panel of experts called upon by the subcommittee for a hearing on “Examining Policies that Inhibit Innovation and Patient Access.” The subcommittee, chaired by Rep. Vern Buchanan (R-Fla.), addresses matters related to payment programs for healthcare, medical delivery systems and related research.
“We all want America to lead the world in medical innovation, and we want Americans to have access to the newest, best groundbreaking treatments as soon as possible,” Rep. Buchanan said. “I hope we can leave this hearing today with a renewed sense of bipartisanship and willingness to work together on policies that protect and enhance innovation.”
Balancing innovation costs with meeting patient needs
“The tradeoff between incentives for innovation and healthcare access for patients is typically framed as an either/or proposition,” Lakdawalla explained. “Either we reward innovators with high prices and deny many patients access to therapies they desperately need, or we make new therapies broadly accessible by limiting their prices, starving innovators of rewards for developing new drugs.”
He then noted how Schaeffer Center research presents potential solutions to this dilemma. “Generous prescription drug coverage can serve as the knife that cuts through this knotty tradeoff,” he said.
“Better lives for patients and their families is the goal,” Lakdawalla emphasized. So instead of paying for all innovations, he said the focus should be on rewarding those companies that “seek out and develop new medicines that help us achieve healthier outcomes.”
Aligning price with value
Such an approach requires measuring the value of new medicines, but Lakdawalla observed that traditional economic analyses, such as quality-adjusted life-years (QALYs), are inaccurate. Instead, he said that Schaeffer Center research has revealed the advantages of value assessment models adhering to the “principle that goods are more valuable to people who have less of them. Analogously, health improvements are more valuable for people with disabilities, terminal illness or other severe disease.”
Such a strategy, he added, “also comports with federal law by avoiding value assessments that discriminate against vulnerable patients with disabilities or terminal illness.”
Yet while the IRA “provides an opportunity to better align price and value for individual drugs,” Lakdawalla said the law needs revision to incorporate “credible, evidence-based and scientifically validated methods for measuring value to patients.”
In response to concerns raised by Kevin Hern (R-Okla.) about the IRA’s policy changes related to rare-disease therapies, Lakdawalla noted that the likely result will be reduced innovation in this category. Therefore, he said, reforms should address the unmet needs of people with rare diseases because, in such cases, “even a relatively modest improvement in health can be quite valuable. And that needs to be accounted for in the way CMS sets maximum fair prices to at least mitigate some of these issues for rare disease where value is at a premium.”
Three-part pricing
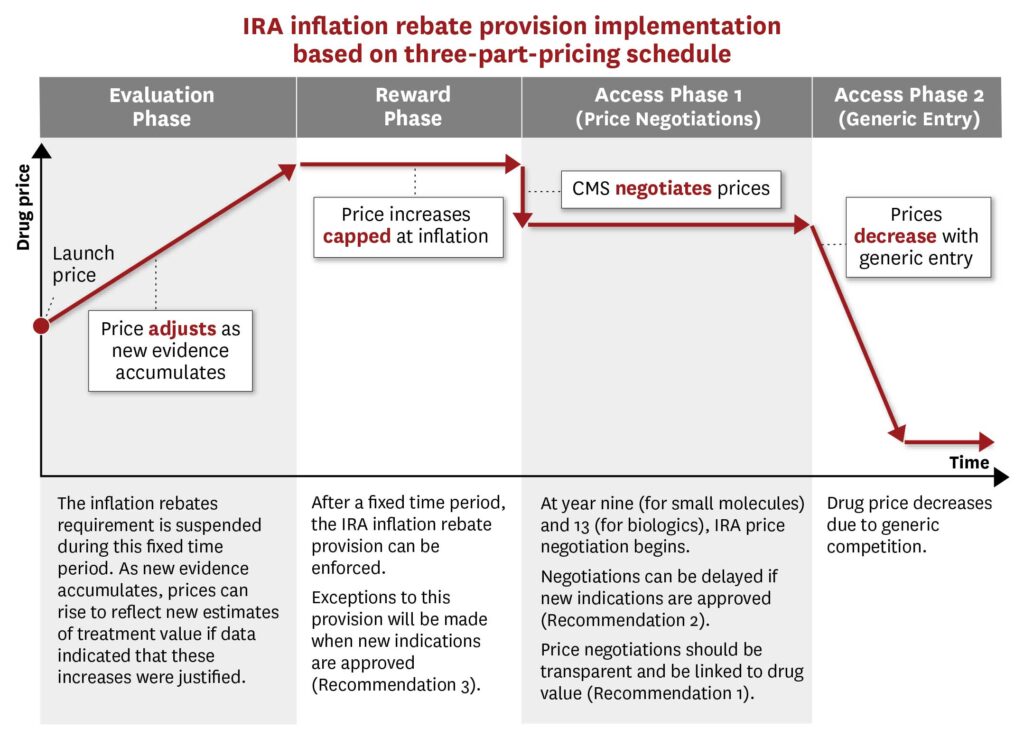
To better align a drug’s cost with its value, Schaeffer Center investigators suggest a three-part-pricing model that starts with an evaluation phase. During this initial period, Lakdawalla explained, the drug would be introduced at “a lower price in exchange for early access to Medicare coverage and the possibility of exemption from IRA inflation rebates if the drug meets prespecified effectiveness benchmarks.”
This would be followed by a reward phase. “If the drug achieves its targets, innovators would be rewarded with a high price,” he said. Otherwise, they would not.
In the final phase, robust generic or biosimilar competition would drive down prices upon the drug’s loss of exclusivity, improving patient access in the long term.
“While there is value in reducing healthcare costs and improving patients’ access to existing drugs in the short term, there is also value in ensuring a continuing stream of innovative therapies for future generations,” Lakdawalla noted. Achieving both aims, however, requires a balanced—and bipartisan approach. The results, however, will be worth the effort, he said.
“By ensuring generous prescription drug insurance, drug prices that reflect the value they deliver and effective competition throughout the pharmaceutical supply chain, we can achieve improved health for Americans today and tomorrow,” Lakdawalla said. “Getting prices right would go a long way toward addressing the different symptoms of our various economic diseases in this market.”
The hearing was webcast live and may be viewed on YouTube. Other panelists were Ted Okon, executive director of the Community Oncology Alliance; Joshua Makower, director of the Stanford Byers Center for Biodesign at Stanford University; Aaron S. Kesselheim, professor of medicine at Harvard Medical School; and Tony Gonzales, national early-stage advisor for the Alzheimer’s Association—who spoke about his battles accessing care after his own Alzheimer’s diagnosis before age 50.
Read Lakdawalla’s testimony in full.
Lakdawalla also serves as Quintiles Chair in Pharmaceutical Development and Regulatory Innovation at the USC Mann School of Pharmacy and Pharmaceutical Sciences and is a professor at the Price School of Public Policy. The Schaeffer Center is a partnership of the Mann School and Price School.
Sign up for Schaeffer Center news



You must be logged in to post a comment.